- Received On: 2021-05-25|
- Accepted On: 2021-06-04|
- Published On: 2021-06-05
| Article Metadata | |||
|---|---|---|---|
| 1 | Submitted Manuscript | PPD/MIN/215/1 | |
| 2 | Cover Letter to Editor | PPD/CLE/215/1 | |
| 3 | Copyright Transfer Letter | PPD/CTL/215/1 | |
| 4 | Authors’ Consent Letter | PPD/ACL/215/1 | |
| 5 | Initial Editorial Screening Report | PPD/IESR/215/1 | |
| 6 | Review Agreement Letter (Reviewer 1) | PPD/RAL/2151/R1 | |
| 7 | Review Agreement Letter (Reviewer 2) | PPD/RAL/2151/R2 | |
| 8 | Manuscript Review Report (Round 1, Reviewer 1) | PPD/MRR/2151/R1.1 | |
| 9 | Manuscript Review Report (Round 1, Reviewer 2) | PPD/MRR/2151/R1.2 | |
| 10 | Revised Manuscript | PPD/MIN/2151R | |
| 11 | Review Response Letter (Round 1) | PPD/RRL/2151/R1 | |
| 12 | Manuscript Review Report (Round 2, Reviewer 1) | PPD/MRR/2151/R2.1 | |
| 13 | Manuscript Review Report (Round 2, Reviewer 2) | PPD/MRR/2151/R2.2 | |
| 14 | Final Editorial Screening Report | PPD/FESR/2151 | |
| 15 | Letter of Acceptance and Acknowledgement | PPD/LAA/2151 | |
| 16 | Accepted Manuscript | PPD/MIN/2151A | |
| 17 | Galley Proof Manuscript | PPD/MIN/GPM/2151 | |
| Request Access | |||
| Supplementary Data | ||
|---|---|---|
| The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request. | ||
| Datasets | Not Provided | Request Availability |
Abstract
Background: A search for novel anxiolytic agents has been the prime target for neuroscientists as the currently available treatment methods have certain limitations like physiological dependence, delayed efficacy and side effects. However, naturally occurring agents are raising hopes. The present study was aimed to evaluate the effects of Ziziphus jujube honey over stress related anxiety behavior in Swiss Albino mice.
Methods: Following Jujube honey administration (at 2g/kg, 4g/kg and 6g/kg; body weight), the experimental mice were employed in open field and hole-cross test apparatus to evaluate neurologic properties and the anxiolytic activities were investigated using elevated plus maze test (EPM). Mice behavior were observed through parameters of square crossing, rearing, grooming, hole crossing, entry and duration in open arm at different time intervals.
Results: At higher doses (6g/kg), Jujube honey was found to possess mild sedative-anxiolytic activity whereas in lower doses (2g/kg & 4g/kg), a non-sedative; anxiolytic potential was observed. Diazepam was effective enough to reduce the locomotor activity and anxiety in all the experiments specially at late phases.
Conclusion: The results demonstrated that the anti-anxiety potential of Jujube honey is dose dependent and could be utilized in broad spectrum in inducing sedation and reducing anxiety in mice. Therefore, jujube honey potentiates scopes for further research as a CNS agent.
Introduction
Anxiety disorders are the most common of the psychiatric disorders and have a high burden of illness (1). Benzodiazepines are the most widely prescribed as the first-line treatments for anxiety disorders because these are considered to be effective and safe, with a rapid onset and favorable tolerability (2). However, concerns have been raised over the years regarding prolonged use of benzodiazepines (3). These drugs have several limitations such as physiological dependence, cognitive and coordination problems, memory and psychomotor impairment and abuse potential. Therefore, there is a need to improve the current drug regimens and find alternative treatments for anxiety disorders (4).
Ziziphus jujube is a fruit that is renowned for both its nutritious and medicinal properties. Jujube is known to possess hypotensive, hypothermic, anti-hyperlipidemia, antihypoxia and anxiolytic properties (5). In many parts of China, jujube is consumed as a medicinal herb that relieves mental tension and calms the mind. It is either prescribed as a single herb or in combination with others to patients suffering from insomnia (6). These neuropharmacological activity of the fruit indicates to a possibility of posing similar potential by other derivatives of this plant.
Honey derived from jujube flower is also known to have therapeutic properties. High concentration of antioxidants are found in jujube honey, compared to other honeys, have been linked to reduce the risk of heart diseases, protect against oxidative stress of free radicals and promote eye health (7). Evaluation of Jujube honey has confirmed its antimicrobial potential against a broad spectrum of bacteria including Staphylococcus aureus (8). Antifungal property of jujube honey was observed against C. albicans (9). Jujube honey has hepatoprotective properties as well and was found to be effective in the suppression of liver injury in alcohol-induced hepatotoxicity in mice (10). Alongside, its high variety in physicochemical nature and mineral content depending on the geographical sources potentiates different intended use for ailments (11).
To evaluate the medicinal as well as nutritional values of jujube honey, many research programs were conducted in many pharmacological fields. However, its neuropharmacological potential has not been explored yet. Therefore, the following study aims to evaluate the ability of Jujube honey to induce sedative and anxiolytic effects in experimental models and examine its potential as an alternative drug to conventional benzodiazepines.
Methods
Drugs and Chemicals
Jujube honey (1 kg) was collected from a cultivated hive in the jujube garden of Gazipur district (23.53°N 92.39°E) in the month of May 2019. This raw honey was stored in an airtight glass jar at room temperature (25˚C). Diazepam was obtained from Square Pharmaceuticals Ltd. For the preparation of test samples, the viscous jujube honey was allowed to pass through a sieve (0.5 mm mesh) to remove non-soluble particles (wax, bee particle, egg, pollen) and other coarse material.
Screening of Physical Properties of Honey
Determination of Moisture and Total Soluble Solids
The moisture content of honey can be deduced from its refractive index (12). To measure the refractive index of honey, a portable honey refractometer (Biobase BK-PRN3, China), having the Brix range of 58 – 92%, thermoregulated at 20˚C and calibrated with distilled water, was used. Temperature correction was applied according to ISO 2173:2003 (13). Total Soluble Solids were inferred from Brix value of honey.
Determination of pH
A pH meter (Biobase pH-10S, China), calibrated at pH 4.01 and 7.00 was used to measure the pH of the sample. The pH measurement was done in triplet where the honey sample was prepared as 10% (w/v) solution in distilled water (14).
Determination of Optical density (OD)
Optical density was determined by using a UV-VIS Spectrophotometer (Biobase BK-UV1800, China) from a 10% (w/v) honey solution in distilled water. Absorbance was taken at 530 nm. Distilled water was used as blank. The method was performed according to the procedure described by El Sohaimy et al. 2015 (15). The obtained absorbance values were compared with standard set by United State Department of Agriculture (1985) (16).
Determination of Honey Density
At first, empty weight of a syringe was taken. After drawing 1 ml honey with the syringe, filled weight was taken. Automatic Electronic Analytical Balance (Biobase BA2004N, China) was used for weight measurement. From the difference of these two weights, mass of the honey was determined. Finally, Density of honey was calculated by using the formula given below as described by Kinoo et al. in 2012 (17):
Density of Honey=(Mass of Honey/Volume of Honey)
Assessment of Sedative-Anxiolytic potential
Acute Toxicity Test
Acute toxicity was evaluated before commencing the in-vivo experimental scheme. Jujube Honey (JH) was orally administered to 20 experimental rodents at the dose of 5g, 7.5g, 10g & 15g per kg of body weight (n=5). The animals were then monitored for the following 72 hours to observe any number of deaths or any unusual symptoms or behavior (18).
Experimental Animal
Female Swiss Albino mice, having 27-32 g of body weight with 45 days of age were selected for the assessment. These animals were habituated with a 12 h light/dark cycle, air ventilation, ambient temperature and ad libitum food and water at animal house of Institute for Pharmaceutical Skill Development and Research. Mice were divided into six groups, each containing 5 animals for control, standard, and test samples, for every experiment to challenge them orally with respective agents.
Group 1: Blank (No gavage), Group 2: Control (Distilled Water), Group 3: Diazepam (1mg/kg), Group 4: JH-2 (2g/kg body weight, in 0.15ml distilled water), Group 5: JH-4 (4g/kg body weight, in 0.15ml distilled water), Group 6: JH-6 (6g/kg body weight, in 0.15ml distilled water).
Experimental Design
Three apparatus were arranged in a continuous series to design a cascade of novelty induced environmental challenge. This method was performed as described by Billah et al., 2019 (19). Mice were placed in Open Field, Hole Cross and Elevated Plus Maze (EPM) sequentially in a row immediately after oral gavage. Behavior of each mice was observed for three minutes in each field. In first interval, mice were placed at open field for the first 3 minutes which was continued with the shifting of mice at hole cross apparatus for the next 3 minutes (however for simplifying, the time denoted as 0 min for hole cross) and at EPM for the last 3 minutes (the time denoted as 0 min for EPM). For each animal, the workflow was repeated in 30, 60, 90 and 120-minute intervals accordingly.
Open Field Test
In this test, mice were placed in an open cubic box, called an open field apparatus which has a dimension of 60x60x60 cm having a tiled (5x5 cm) floor alternatively colored black and white. This method was carried out as described by Rayhan et al. 2019 (20). Sedative-anxiolytic activities were evaluated by observing parameters such as number of squares crossed, grooming and rearing.
Hole Cross Test
After placing in a Hole Cross apparatus, mice were freely allowed to cross a 3 cm hole made on a partition at 7 cm floor height which divided the box of 30x20x14 cm into two equal compartments. During the observation, number of holes crossed was counted as a parameter of exploratory behavior as described by Nawrin et al. 2015 (21).
Elevated Plus Maze Test
In this experiment, mice were placed in the center of the maze and allowed to move in any direction. It has mirror-designed plus shaped two open arms intersecting with two closed arms each having a length of 14 cm and width of 5 cm. The close arm has wall height of 14 cm. Duration and entry in open and closed arms were observed as parameters to assess the anxiolytic potential. This test was executed as described by Hawiset et al., 2011 (22).
Statistical Analysis
Statistical analysis of data was performed by using the method of one-way analysis of variance (ANOVA), followed by Dunnett’s t-test by using SPSS 24 for windows. The obtained results were compared with the control group. P values < 0.05, 0.01 and 0.001 were accepted as statistically significant.
Results
Physical Properties of Jujube Honey
The assessment of physicochemical properties of Jujube honey reveals that the honey is physically light brown in color and acidic in nature (pH 5.6). Optical density measured at 530 nm was recorded 0.381 which indicates white to extra light amber color according to USDA guideline. The moisture content was found 18.6 g/100 g of honey which was within the internationally accepted limit (NMT 21 g/100 g).
Table 1. Physicochemical properties of jujube honey.
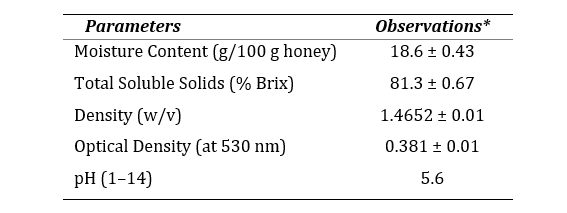
*All the methods performed in triplicate. Data represented as mean ± SEM (n=3). Compared with USDA guideline for honey quality, 1985.
Acute Toxicity Study
Higher doses of jujube honey resulted in no death within the observation period. Moreover, no physical abnormalities or unusual behavior was recorded in the treated groups.
Open Field Test
The results of the open field test are shown in Figure 1(a-c). The CNS activity of Jujube honey (JH) was evaluated by its effect on locomotion of the test animal. In this test, jujube honey showed dose dependent decrease in movements. Fig. 1a demonstrated that jujube honey at higher dose (6mg/kg b.w.) reduced the number of square crossing activity immediately after administration (0 min, 60.8) and at final observation period (120 min, 9.4) in comparison to that of diazepam (1mg/kg b.w.). There was no significant change in activity observed up to 90 minutes for JH-6 until a rapid fall in numbers recorded at 120-minute interval. On the contrary, diazepam was found to reduce the activity gradually
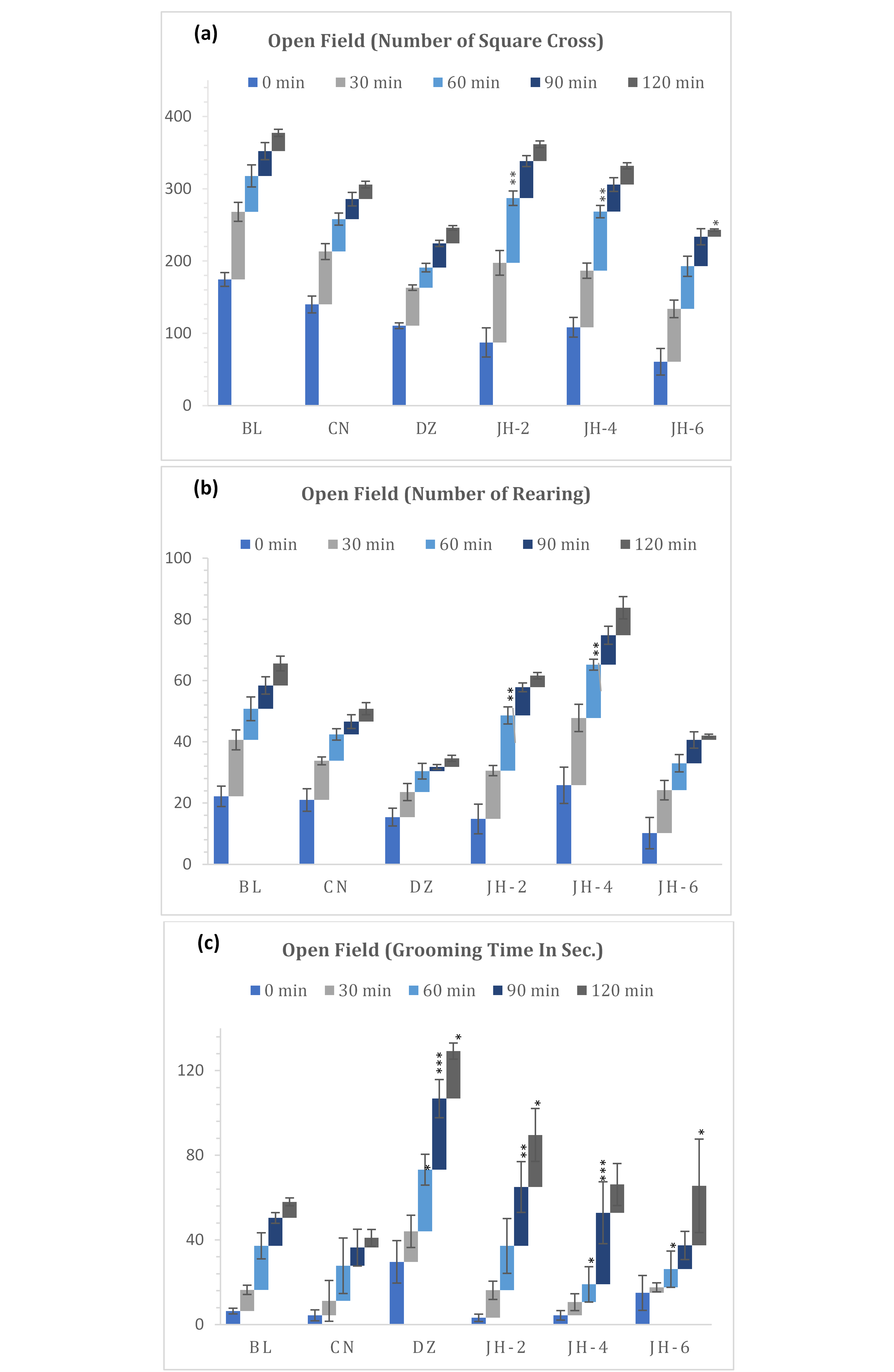
Fig. 1(a-c): Distance travel, rearing & grooming activities in open field test. BL=Blank, CN=Control, DZ=Diazepam (1mg/kg), JH-2/4/6=Jujube Honey at 2 gkg-1/4 gkg-1/6 gkg-1. Data represented as mean ± SEM, (n = 5); *p < 0.05, **p < 0.01, ***p < 0.001; Dunnett t-test (two sided) treated one group as control (water) and compared all other groups against it.
from early (0 min, 110.6) to late interval (120 min, 21.6) of the administration. In addition, JH-2 and JH-4 were found with higher locomotion in all intervals. Moreover, it was noted that all the groups showed minimum response at late phase compared to that of their response at early phase.
From the observations of rearing activity (Figure 1b), it was found that diazepam decreased the activity gradually from 30 minutes to 120 minutes (8.2 to 2.8) compared to that of control group (12.8 to 4.2). JH-6 followed similar pattern in showing activity (14.0 to 1.4) and demonstrated a significant difference from the control. Like square crossing, lower doses (JH-2 and JH-4) were found to increase the rearing activities at early interval and showed mild decrease over time.
Grooming behavior of test mice was also assessed. JH treated mice showed significant deficits in overall grooming behavior compared to the diazepam treated ones. The reference drug treated group displayed a significant increase in grooming from starting point to last observation period. As for the JH treated group, data indicated a dose dependent reduction in grooming duration exhibited by mice. At the dose of 2mg/kg b.w. the activity was observed higher in comparison to higher doses. JH-4 and JH-6 were found with lesser grooming time from 0 to 60 minutes and greater response in 90 and 120 minutes.
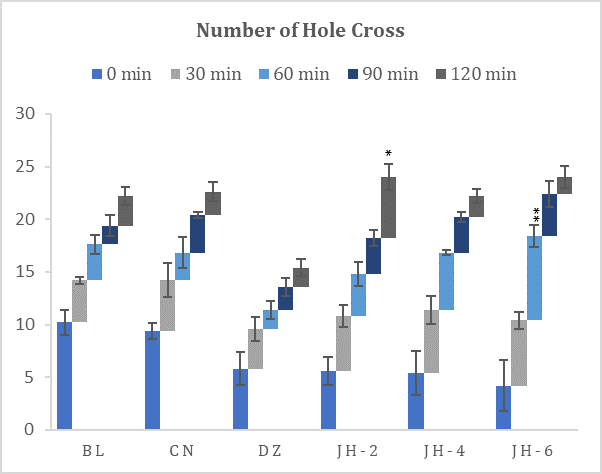
Fig. 2: Number of hole crossed in hole cross test. BL=Blank, CN=Control, DZ=Diazepam (1mgkg-1), JH-2/4/6=Jujube Honey at 2 gkg-1/4 gkg-1/6 gkg-1. Data represented as mean ± SEM, (n = 5); *p < 0.05, **p < 0.01; Dunnett t-test (two sided) treated one group as control (water) and compared all other groups against it.
Hole Cross Test
The results (Fig. 2) demonstrated that Jujube honey given at all doses increased the number of holes crossed by the mice, in contrast to the control group. There were no significant differences observed against difference doses and by different time intervals. On the contrary, diazepam decreased the locomotor activity of the experimental mice. The suppressive effect was observed 30 minutes after oral administration. However, the decrements with JH doses are dose-dependent. The locomotor activity was maximally suppressed at 120 min.
Elevated Plus Maze Test
The oral dosing (6 g/kg) of the Jujube honey exhibited a substantial increase in the entry and duration into the open arms of the elevated plus-maze (EPM) whereas diazepam significantly decreased the numbers of entries and the duration (fig. 3a-3b). With JH-6 dose, the percentage of time spent in the open-arm peaked from 60 minutes to 120 minutes interval.
Discussion
The quality and nature of honey is dependent on its physicochemical properties. Since jujube honey has low moisture content, bacteria have less water to undergo fermentation so bacterial growth is restricted and the quality of honey is not compromised (23). As a measure of higher stability on storage, more than 80% of TSS signified a high grade of honey (24). Acidic nature of this honey could mean a high organic or amino acid content (25).
In this study, the anxiolytic effects of Jujube honey were studied in different neurobehavioral models and compared with the standard diazepam. Open field and hole-cross tests were used to assess locomotor activity whereas elevated plus maze was selected to examine the direct anxiolysis upon different doses of JH. The experimental models were arranged in a cascade to deploy the rodents in each field on the same event so as to minimize the time-dependent and personality-dependent variance which are considered major limitations of these kinds of behavioral analysis. For example, a major drawback with hole-cross model is that the mice get habituated with the environment and lose their curiosity to explore due to repeated exposure to the same environment, this may lead to a decrease in the ambulatory activity (26). However, the experimental design resulted in over-stress for rodents due to excessive handling and it was perceived as an advantage to create stress-induced anxiety model.
Stress-induced anxiety is predominantly caused by increased proinflammatory cytokines/chemokines, oxidative stress markers, gut microbial content and tissues which activate blood-borne immune cells. Altogether these stimuli send signal to blood-brain-barrier (BBB) for their cognate receptors’ activation on endothelial cells, pericytes and perivascular microglia. As a consequence, disruption of BBB occurs through a series of pathophysiological progressions in the perivascular neurons and endfeet astrocytes where NF-κB, NLRP3, and Nrf2 signaling pathways plays key role (27).
Open Field test is a standard behavioral model that is used to evaluate the locomotor activity and anxiety behavior of the rodents (28). Tendency to avoid open spaces, preference for peripheral areas were observed through number of peripheral square crossing. Other behaviors associated with treatments such as grooming and rearing were noted. An increase in the locomotor function indicates a state of alertness, while the opposite is an index of sedation (29). As shown in Figure 1, mice treated with diazepam and JH (6g/kg) when compared to control group, showed significant decrease in ambulatory activity which indicated to their mild sedation potential. Like square crossing, rearing activities were also decreased by these two test groups. Rearing, the vertical movement demonstrates exploration and is considered as an indicator of anxiolysis (19). However, JH-2 and JH-4 significantly increased both activities. Together the findings demonstrated non-sedative though anxiolytic activity by the lower doses. The anxiolytic action was also evident by increased grooming response by lower doses of honey which gradually decreased with increment of the doses. With standard diazepam, the mice demonstrated highest grooming time. An abnormal increase in grooming suggests low-stress, whereas when animals are experiencing excessive stress grooming is diminished significantly (30).
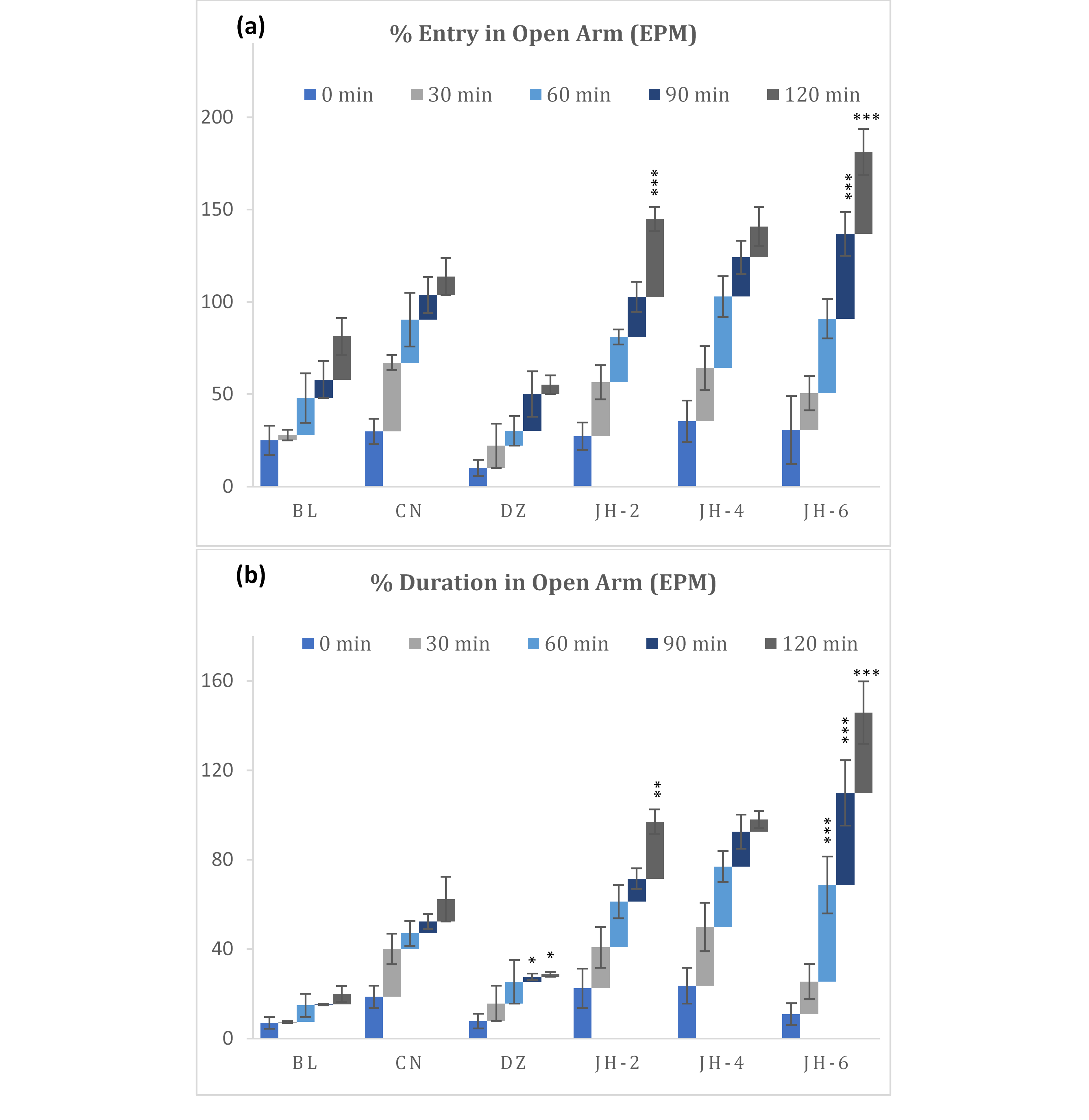
Fig. 3 (a-b): Percentage entry and duration in open arm in elevated plus maze test. BL=Blank, CN=Control, DZ=Diazepam (1mgkg-1), JH-2/4/6=Jujube Honey at 2 gkg-1/4 gkg-1/6 gkg-1. Data represented as mean ± SEM, (n = 5); *p < 0.05, **p < 0.01, ***p < 0.001; Dunnett t-test (two sided) treated one group as control (water) and compared all other groups against it.
The hole-cross test is quite similar to OF test because it also evaluates the spontaneous and exploratory activity of mice which is proportional to anxiolysis (31). In this test, all the groups of mice treated with honey crossed more holes when compared to the group treated with diazepam which presented anxiolytic-like activity.
The elevated plus maze is a promising behavioral test for evaluating anxiety-like behavior. In the EPM test, substances that have anxiolytic activity cause an increase number of entries in open arms and induce mice to spend more time whereas anxiogenic substances decrease open arm exploration (32). Administration of JH displayed anxiolytic-like effects with increasing dose, as evidenced by increased number of entries and time spent in open arms. This indicates decreased anxiety compared to control group. As for diazepam, the percentage of entries and time spent in open arms were far less than that of tested samples. This may be due to the mild sedative-hypnotic activity commonly exhibited by the classic benzodiazepines like diazepam.
The reason behind assessing the effects against benzodiazepines is that these are the drug of choice for treatment of anxiety. However, continuous use of these drugs is associated with side effects like withdrawal effects, psychomotor impairment and physical dependence/ addiction (33). The action of benzodiazepines is mediated via positive allosteric modulation of the GABAA (γ-aminobutyric acid type A) receptor complex. By binding to alpha-gamma subunit interface it facilitates neuronal chloride-ion influx to hyperpolarize postsynaptic membranes (34). The anxiolytic effect is obtained through a stimulation of GABAA receptor in the limbic system, thalamus, hypothalamus, and cerebral cortex at α2/α3 subunit isoforms. This produces relaxing effects and enables the anxiolytic process (35). A decrease in ambulatory and exploratory behaviors also exerts such types of effect.
Jujube honey contains minerals in abundance (potassium, calcium, sodium, and magnesium) (11). Alongside it is reach in gallic acid, protocatechuic acid, p-hydroxybenzoic acid, chlorogenic acid, caffeic acid, p-coumaric acid, ferulic acid and ellagic acid. The anti-anxiety activity of the honey can be correlated with its immense antioxidant properties (10). However, further investigation is recommended for concluding with the responsible compounds.
Conclusion
In summary, the results suggest that Jujube honey possesses anxiolytic activity at lower doses and sedative-anxiolytic effects at higher doses. Thus, Zizyphus Jujube honey could be a promising alternative therapy to the conventional benzodiazepines, for treatment anxiety as well as insomnia. However, further research is needed to understand the mechanisms underlying the observed pharmacological activities.
Abbreviations
JH: Jujube Honey; OF: Open Field; HC: Hole Cross; EPM: Elevated Plus Maze; b.w.: body weight; USDA: United State Department of Agriculture.
Acknowledgments
The present study was supported and carried out in the Pharmacology lab of Institute for Pharmaceutical Skill Development and Research, Bangladesh. Authors are grateful to the institution for providing such opportunity to contribute to health science.
Authors’ Contributions
This work was carried out in collaboration between all authors. Authors MMB and KN designed coordinated and supervised the project. MAR performed in vivo experiments and prepared the graphical presentations. NJV participated in the assessment of physical properties and prepared the manuscript. FA participated in the experiment and critically revised the manuscript. AH participated in interpretation of data to reach a scientific discussion. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All experiments associated with animal handling were performed in accordance with the Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection adopted by the institutional guideline for animal handling (Ref. no. IPSDRLAB/AHCP/01/18).
The experimental design was authorized by the Institutional Ethical Committee Clearance (Ref. No. IPSDRLAB/IECC/18/19) from the Institute for Pharmaceutical Skill Development and Research, Bangladesh (project approved on 12/05/2019).
Consent for Publication
Not applicable.
Competing Interests
All authors agreed on the article before submission and had no conflict of interests.
References
- Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19(2):93-107. DOI PubMed PMC Google Scholar
- Tanguay Bernard M-M, Luc M, Carrier J-D, et al. Patterns of benzodiazepines use in primary care adults with anxiety disorders. Heliyon. 2018;4(7). DOI PubMed PMC Google Scholar Link
- Dell’osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28(1):7-20. DOI PubMed PMC Google Scholar Link
- Christmas D, Hood S, Nutt D. Potential novel anxiolytic drugs. Curr Pharm Des. 2008;14(33):3534-3546. DOI PubMed Google Scholar
- Jiang J-G, Huang X-J, Chen J. Separation and purification of saponins from Semen Ziziphus jujuba and their sedative and hypnotic effects. J Pharm Pharmacol. 2007;59(8):1175-1180. DOI PubMed Google Scholar Link
- Chen J, Liu X, Li Z, et al. A Review of Dietary Ziziphus jujuba Fruit (Jujube): Developing Health Food Supplements for Brain Protection. Evid Based Complement Alternat Med. 2017;2017:3019568. DOI PubMed Google Scholar Link
- Ullah R, Alsaid MS, Shahat AA, et al. Antioxidant and Hepatoprotective Effects of Methanolic Extracts of Zilla spinosa and Hammada elegans Against Carbon Tetrachloride Induced Hepatotoxicity in Rats. Open Chemistry. 2018;16(1):133-140. DOI Google Scholar Link
- Fahim H, Dasti JI, Ali I, Ahmed S, Nadeem M. Physico-chemical analysis and antimicrobial potential of Apis dorsata, Apis mellifera and Ziziphus jujube honey samples from Pakistan. Asian Pac J Trop Biomed. 2014;4(8):633-641. DOI PMC Google Scholar Link
- Ansari MJ, Al-Ghamdi A, Usmani S, et al. Effect of jujube honey on Candida albicans growth and biofilm formation. Arch Med Res. 2013;44(5):352-360. DOI PubMed Google Scholar Link
- Cheng N, Du B, Wang Y, et al. Antioxidant properties of jujube honey and its protective effects against chronic alcohol-induced liver damage in mice. Food Funct. 2014;5(5):900-908. DOI PubMed Google Scholar Link
- Zhou J, Suo Z, Zhao P, et al. Jujube honey from China: physicochemical characteristics and mineral contents. J Food Sci. 2013;78(3):C387-394. DOI PubMed Google Scholar Link
- Diacu E, Tantaveanu E. Determination of Moisture Content and its Correlation with other Parameters in Honey Quality Control. undefined. Published online 2007. Accessed May 24, 2021. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.567.9436&rep=rep1&type=pdf Google Scholar Link
- International Standard Organisation. Fruit and vegetable products—determination of solu-ble solids—refractometric method. 2nd edition, International Organisation for Standardisation, Geneva, Switzerland, 1998. Accessed May 24, 2021. https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/03/58/35851.html Link
- Iftikhar F, Mahmood R, Islam N, Sarwar G, Masood MA, Shafiq H. Physicochemical Analysis of Honey Samples Collected from Local Markets of Rawalpindi and Islamabad, Pakistan. American Journal of Biochemistry. 2014;4(2):35-40. Google Scholar Link
- El Sohaimy SA, Masry SHD, Shehata MG. Physicochemical characteristics of honey from different origins. Annals of Agricultural Sciences. 2015;60(2):279-287. DOI Google Scholar Link
- United State Department of Agriculture. United States standards for grades of extracted honey. United State Department of Agriculture, Washington DC, 1985. Accessed May 24, 2021. Google Scholar Link
- Kinoo MS, Mahomoodally MF, Puchooa D. Anti-Microbial and Physico-Chemical Properties of Processed and Raw Honeys of Mauritius. Advances in Infectious Diseases. 2012;2(2):25-36. DOI Google Scholar Link
- Billah MM, Chowdhury AS, Nawrin K, Mostaq S, Rayhan MdA, Tushar RR. Serotonergic and noradrenergic response of ethanol extract; opioidergic response of ethyl acetate extract of Dicranopteris linearis L. leaf. Clinical Phytoscience. 2021;7(1):25. DOI Google Scholar Link
- Billah MM, Rayhan MA, Yousuf SA, Nawrin K, Rayhan J, Khengari EM. A Novel Integrated (OF-HC-EPM) Approach to Study Anxiety Related Depressive Behavior in Mice Model: A Comparison of Neuro Standards. Advances in Pharmacology and Pharmacy. 2019;7(3):39-48. DOI Google Scholar Link
- Rayhan MA, Yousuf SA, Rayhan J, Khengari EM, Nawrin K, Billah MM. Black seed honey—a power-ful ingredient of prophetic medicine; its neurophar-macological potential. J Apither 2019. 5(2):18–26; DOI Google Scholar Link
- Nawrin K, Billah MM, Jabed MSU, Roy A, Ahmad AKMR, Islam MdN. Antipyretic, Antidiabetic, Thrombolytic and CNS Depressant Potential of Ethanol Extract of Crotalaria Verrucosa L. Leaves. Am J Biomed Sci. Published online October 2015:198-204. DOI Google Scholar Link
- Hawiset T, Muchimapura S, Wattanathorn J, Sripanidkulchai B. Screening Neuropharmaco¬logical Activities of Kaempferia parviflora (Krachai Dam) in Healthy Adult Male Rats. American Journal of Applied Sciences. 2011;8(7):695-702. DOI Google Scholar Link
- Khan M, Islam Z, Chowdhury AS, Yousuf SA, Amin MR, Rayhan MA. Wild Honey Facilitates Antibacterial Efficacy of Penicillin and Amoxicillin-Clavulanic Acid. Journal of Apitherapy. 2020;7(2):22–30. DOI Google Scholar Link
- Nyau V, Mwanza EP, Moonga HB. Physico- chemical qualities of honey harvested from different beehive types in Zambia. African Journal of Food, Agriculture, Nutrition and Development. 2013;13(2). DOI Google Scholar Link
- da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016;196:309-323. DOI PubMed Google Scholar Link
- Moniruzzaman M, Mannan MA, Hossen Khan MF, Abir AB, Afroze M. The leaves of Crataeva nurvala Buch-Ham. modulate locomotor and anxiety behaviors possibly through GABAergic system. BMC Complement Altern Med. 2018;18(1):283. DOI PubMed Google Scholar Link
- Welcome MO. Cellular mechanisms and molecular signaling pathways in stress-induced anxiety, depression, and blood-brain barrier inflammation and leakage. Inflammopharma-cology. 2020;28(3):643-665. DOI PubMed Google Scholar Link
- Bagewadi HG, AK AK, Shivaramegowda RM. An Experimental Study to Evaluate the Effect of Memantine in Animal Models of Anxiety in Swiss Albino Mice. J Clin Diagn Res. 2015;9(8):FF01-FF05. DOI PMC Google Scholar Link
- Rahman MM, Uddin ME, Islam AMT, Chowdhury MAU, Rahman MA. CNS Depressant and Antinociceptive Effects of Different Fractions of Pandanus foetidus Roxb. Leaf Extract in Mice. Malays J Med Sci. 2015;22(3):33-40. PubMed Google Scholar Link
- Fedotova J, Barishpolec V, Zulli A, Büsselberg D, Gaspar L, Kruzliak P. Effects of acute or chronic administration of novel 3,4-dimethoxy-phenylethylamine derivates on anxiety-like behavior. Am J Transl Res. 2015;7(11):2462-2473. PubMed Google Scholar
- Liebsch G, Montkowski A, Holsboer F, Landgraf R. Behavioural profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour. Behav Brain Res. 1998;94(2):301-310. DOI PubMed Google Scholar Link
- Bhatt S, Mahesh R, Devadoss T, Jindal AK. Anxiolytic-like effect of (4-benzylpiperazin-1-yl)(3-methoxyquinoxalin-2-yl)methanone (6g) in experimental mouse models of anxiety. Indian J Pharmacol. 2013;45(3):248-251. DOI PubMed Google Scholar Link
- Nagaraja TS, Mahmood R, Krishna V, Thippeswamy BS, Veerapur VP. Evaluation of Anxiolytic effect of Erythrina mysorensis Gamb. in mice. Indian J Pharmacol. 2012;44(4):489-492. DOI PMC Google Scholar Link
- Calcaterra NE, Barrow JC. Classics in Chemical Neuroscience: Diazepam (Valium). ACS Chem Neurosci. 2014;5(4):253-260. DOI PMC Google Scholar Link
- Zakusov VV, Ostrovskaya RU, Kozhechkin SN, Markovich VV, Molodavkin GM, Voronina TA. Further evidence for GABA-ergic mechanisms in the action of benzodiazepines. Arch Int Pharmacodyn Ther. 1977;229(2):313-326 PubMed Google Scholar
Update History
| Revision Number | Date | Details Of Changes |
|---|---|---|
| 01 | 2021-06-05 | Original Article; published at its accepted version (Reference Number: PPD/MIN/2151A) |
 Pharmacotherapy & Pharmascience Discovery
Pharmacotherapy & Pharmascience Discovery
