- Received On: 2021-05-30|
- Accepted On: 2021-06-12|
- Published On: 2021-06-13
| Article Metadata | |||
|---|---|---|---|
| 1 | Submitted Manuscript | PPD/MIN/216/1 | |
| 2 | Cover Letter to Editor | PPD/CLE/216/1 | |
| 3 | Copyright Transfer Letter | PPD/CTL/216/1 | |
| 4 | Authors’ Consent Letter | PPD/ACL/216/1 | |
| 5 | Initial Editorial Screening Report | PPD/IESR/216/1 | |
| 6 | Review Agreement Letter (Reviewer 1) | PPD/RAL/2161/R1 | |
| 7 | Review Agreement Letter (Reviewer 2) | PPD/RAL/2161/R2 | |
| 8 | Manuscript Review Report (Round 1, Reviewer 1) | PPD/MRR/2161/R1.1 | |
| 9 | Manuscript Review Report (Round 1, Reviewer 2) | PPD/MRR/2161/R1.2 | |
| 10 | Revised Manuscript | PPD/MIN/2161R | |
| 11 | Review Response Letter (Round 1) | PPD/RRL/2161/R1 | |
| 12 | Manuscript Review Report (Round 2, Reviewer 1) | PPD/MRR/2161/R2.1 | |
| 13 | Manuscript Review Report (Round 2, Reviewer 2) | PPD/MRR/2161/R2.2 | |
| 14 | Final Editorial Screening Report | PPD/FESR/2161 | |
| 15 | Letter of Acceptance and Acknowledgement | PPD/LAA/2161 | |
| 16 | Accepted Manuscript | PPD/MIN/2161A | |
| 17 | Galley Proof Manuscript | PPD/MIN/GPM/2161 | |
| Request Access | |||
| Supplementary Data | ||
|---|---|---|
| The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request. | ||
| Datasets | Not Provided | Request Availability |
Abstract
Background: In different parts of the world, citrus fruit peels are considered potential sources for natural anti-inflammatory agents. The present study was designed to evaluate the anti-inflammatory potential of Citrus. sinensis fruit peel in search for a natural alternative medicine.
Methods: In vitro inflammation was modeled through egg albumin denaturation, human erythrocyte membrane destabilization- in hypotonic and thermal conditions; treated with the aqueous peel extract at various concentrations - 31.25, 62.5, 125, 250 and 500 μg/ml and compared with the same concentrations of the reference acetyl salicylic acid (ASA). Half maximal inhibitory concentration (IC50) was determined from the dose-response curve. In addition, phytochemical and physicochemical properties were also assessed.
Results: In all experiments, IC50 of the test sample was achieved at higher concentrations (>200 μg/ml). However, in hypotonic and hyperthermic conditions, IC50 was attained at much higher concentrations (>250 μg/ml). ASA showed greater responses in inhibition in comparison to that of the sample. The qualitative phytochemical analysis exhibited that the aqueous extract contained protective components like tannin, phenol, flavonoid, reducing sugar, protein, carbohydrate and steroid.
Conclusion: The demonstration of anti-protein denaturation and erythrocyte membrane stabilization indicated a significant potential of the aqueous fruit peel extract as natural anti-inflammatory agent which generated the context for further in-depth investigation
Introduction
Inflammation refers to as a mammalian body defense response at local sites of tissue or cell damage (1). Inflammation occurs to eradicate or halt the spread of tissue hazards and manifests by edema through an increased tissue permeability and endothelial leukocyte influx of blood into the interstitial space (2). Though being a natural response to tissue damage, it is often uncontrolled in allergies such as anaphylactic shock and asthma; or in chronic autoimmune diseases, more commonly in Crohn’s disease and rheumatoid arthritis (3). Many of the diseases exhibit common non-specific manifestations through inflammation. Inflammatory cells are packed with various endogenous mediators like prostaglandins, histamine, serotonin, bradykinin etc., among which PG is found almost universally to direct and modulate tissue and cellular responses associated with inflammation (4,5). Any anti-inflammatory agents demonstrate their therapeutic efficacy by suppressing the action or blocking the synthesis of these mediators. NSAIDs, opioids, steroids are the most commonly used anti-inflammatory drugs in modern practice, however, are associated with severe side effects like ulceration, gastrointestinal bleeding, renal toxicity etc., particularly in arthritic and chronic inflammatory disease patients (6–8). On the other hand, the ethnopharmacological use of naturally occurring agents raises hope in reducing the side effects and providing lot more beneficial effects alongside. Thus, the need for searching and developing newer anti-inflammatory medicine is eminent and draws attention from the research scientists.
Several studies have confirmed that citrus fruits showed anti-inflammatory potential (9–13). Citrus sinensis, from the genus Citrus and family Rutaceae, have been acknowledged for its peel to exhibit antibacterial, antioxidant, antithyroidal, hypoglyce-mic, antidiabetic, anti-hypercholesterolemic and anti-inflammatory properties in different solvent extracts (14–17). Moreover, it demonstrated protective role against oxidative stress and gastric ulcer (18). Though being a widely popular and among the mostly consumed fruit, C. sinensis fruit peel is generally discarded as a wastage. This potentiates the opportunity for developing an easily obtainable, abundant and cheap source for natural medicine. While much literature has been published on the peel extract of C. sinensis fruit, no study design included in vitro assessment of anti-inflammatory properties with its aqueous extract. Therefore, the present study was aimed to investigate the in vitro anti-inflammatory potential of C. sinensis fruit peel aqueous extract in different concentrations and in different simulated biological conditions and finally, compare its efficacy with reference drug.
Methods
Collection and Preparation of Sample
Fresh ripe Citrus sinensis (Malta) fruit was purchased from a local market at Dhaka, Bangladesh in March, 2020. The fruits were washed to remove the surface debris under running tap water followed by a second wash on distilled water. Peels of the fruits were removed and approximately 500g of the isolated peel was collected for extraction procedure using distilled water (17). Acetyl Salicylic Acid (Aspirin) was obtained from Renata Limited.
Qualitative Phytochemical and Physicochemical Analysis
The qualitative analysis of the phytochemical composition of the extract was carried out using the standard procedures (19). Alongside, the physico-chemical screening was performed by following the established methods (20).
In Vitro Anti-Inflammatory Activities
The following in vitro tests were executed for assessment of anti-inflammatory activity of crude aqueous extract of C. sinensis fruit peel.
Anti-Protein Denaturation Test
The experiment was conducted according to the protocol described by Obiang et al., 2021 (21). A reaction mixture was made to determine the protective capacity on albumin denaturation. The solution contained 0.1ml of fresh chicken egg albumin, 0.9 ml of phosphate buffered saline (pH 6.4) and 1.9 ml of different concentrations of peel extract so as to generate final concentrations - 31.25, 62.5, 125, 250 and 500 μg/ml. The mixtures were allowed for an incubation at 37°C for 20 min followed by heating at 70°C for 5 min. Finally, absorbances were measured at 660 nm with Biobase UV/VIS-1800 spectrophotometer. Aspirin at the same concentrations served as the reference whereas distilled water was used as control and similarly processed for measuring the absorbances. The concentration at which 50% maximal inhibition recorded, was denoted as IC50, calculated from the below formula and determined from the dose response curve:
% inhibition = { (Sample Abs-Control Abs)/Control Abs } X 100
Where, Abs = absorbance.
HRBC Membrane Stabilization Assay
The experiment was carried out to determine the suppressive potential of Citrus sinensis peel extracts on simulated biological inflammation (21,22). A volume of 3ml of human fresh blood was drawn from healthy volunteers and was mixed with equal volume of 0.9% normal saline before subjected to a centrifuge at 3000 rpm for 10 minutes at room temperature. The collected packed cells were washed three times with 10mM sodium phosphate buffer solution (isotonic, pH 7.4) and a 10% v/v suspension was reconstituted. The buffer solution was constituted as 0.2 g of NaH2PO4, 1.15 g of Na2HPO4 and 9 g of NaCl in 1 L of distilled water. For assessing the protecting effect of peel extract against hemolysis, 0.5 ml of the HRBC suspension was mixed with 1ml of phosphate buffer (pH 7.4), 2 ml of hyposaline (0.2% NaCl) and 1 ml of various concentrations of the extract to generate final concentrations of 31.25, 62.5, 125, 250 and 500 μg/ml. Similar processing were applied for the standard and negative control. After an incubation at 37°C for 30 min followed by a centrifugation at 3000 rpm for 20 min, the supernatant was collected and estimated for hemoglobin content by UV-VIS spectrophotometer at 560 nm. The percentage of hemolysis was measured by considering the hemolysis exhibited by the control group as 100%. The HRBC membrane stabilization or the percentage of protection was calculated using the formula:
% protection = 100 – (OD of Sample/OD of Control ) ×100
Where, OD = Optical Density.
Temperature Induced Haemolysis Assay
In this experiment isotonic phosphate buffer solution was used to dissolve the test extracts (23). A reaction mixture containing the test extracts (5ml) in different concentrations and 10% v/v HRBC suspension (0.1ml) was obtained to get the end concentrations as 31.25, 62.5, 125, 250 and 500 μg/ml. Normal saline and Aspirin at the same concentrations served as control and standard respectively. Two sets of tubes were arranged accordingly – one was kept at 54°C for 20min in a thermoregulated water bath whereas the other set was kept at 4°C for 20min in a medical refrigerator. After the incubation period these tubes were centrifuged at 3000rpm for 3min. The supernatant was collected to determine the hemoglobin content spectrophotometrically at 540nm. Inhibition of haemolysis was calculated in percentage by the following formula:
% Inhibition of haemolysis = {1 - (OD2-OD1/OD3-OD1) }X 100
Where, OD1 = Test Sample Unheated; OD2 = Test Sample Heated and OD3 = Control Sample Heated.
Tonicity Induced Haemolysis Assay
In this experiment, the extracts in different concentrations were dissolved in hypotonic (0.2% sodium chloride) solution in one hand and in isotonic solution (0.9% sodium chloride) in other hand (24). Like hyperthermia induced hemolysis test, both of these reaction mixtures were constituted of test extracts (5ml) in different concentrations and 10%v/v HRBC suspension (0.1ml) to draw the final concentrations as 31.25, 62.5, 125, 250 and 500 μg/ml. Aspirin was used as the standard and distilled water served as the control. Both sets of tubes were allowed for an incubation at 37°C for 1hr followed by a centrifugation at 3000rpm for 3min. The supernatant was collected and its haemoglobin content was determined by running the collected supernatant in spectrophotometer at 540nm. Inhibition of haemolysis was calculated in percentage by the following formula:
% Inhibition of haemolysis = {1 - (OD2-OD1/OD3-OD1) }X 100
Where, OD1 = Test sample in isotonic solution; OD2 = Test sample hypotonic solution and OD3 = Control sample in hypotonic solution.
Statistical Analysis
Data were presented as mean ± standard error of mean (SEM) (n = 5). Linear regression was performed to determine the half maximal inhibitory concentration (IC50). The difference between the experimental and control groups was carried out using one-way analysis of variance (ANOVA) in SPSS (v.26) followed by a sample T-test. p < 0.05 was considered significant for any differences.
Results
Qualitative Phytochemical and Physicochemical Analysis
The qualitative investigation of phytochemical composition exhibited that the aqueous extract of C. sinensis fruit peel is composed of tannin, phenol, flavonoid, reducing sugar, protein, carbohydrate and steroid as shown in Table 1.
Table 1. Phytochemical compositions of CSAQ
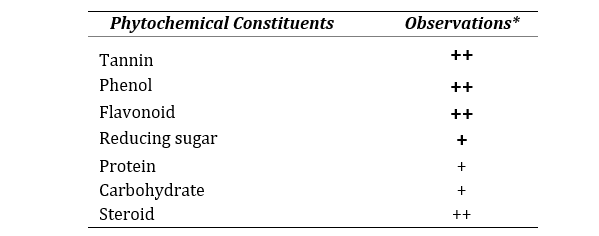
*All the methods performed in triplicate. Data represented as mean ± SEM (n=3).
The physicochemical assessment displayed that the aqueous extract of the peel is acidic in nature, possess high soluble content and low moisture as shown in Table 2.
Table 2. Physicochemical properties of CSAQ
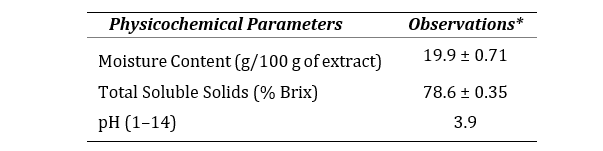
*All the methods performed in triplicate. Data represented as mean ± SEM (n=3).
Anti-Protein Denaturation Test
Percentage inhibition of egg albumin denaturation by the standard and peel extract was shown in Figure 1. The figure demonstrated a gradual increase in inhibition for both ASA and CSAQ with the increase of concentrations. Maximum protection was observed at 500 µg/ml for ASA (94.32±4.32%) and CSAQ (88.29±10.41%). The IC50 was found at 116.26 µg/ml for ASA and 208.05 µg/ml for CSAQ.
HRBC Membrane Stabilization Assay
The findings of HRBC membrane stabilization assay were depicted in Figure 2, where the activities of ASA and CSAQ were expressed as the percentage of protection against RBC lysis. ASA was found to reduce the membrane lysis up to 50% in 82.26 µg/ml whereas, CSAQ reached with similar activity at much higher concentration 263.16 µg/ml. ASA showed greater significance in haemolysis at 500 µg/ml.
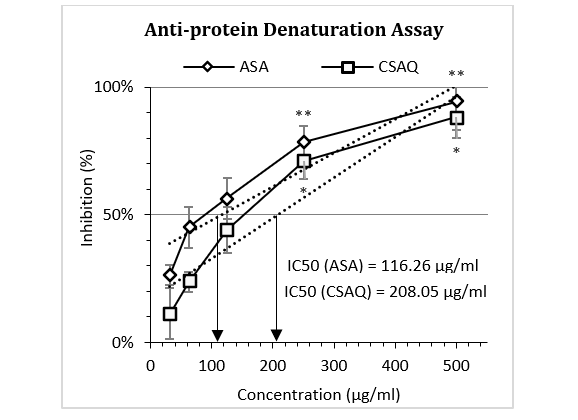
Figure 1: Percentage of inhibition of egg albumin denaturation.
Data represented as mean (%) ± SEM, (n = 5); IC50 calculated from linear regression; *p < 0.05, **p < 0.01; Sample T-test treated one group as control and compared all other groups against it. X-axis, applied concentrations were 31.25; 62.5, 125, 250 and 500 µg/ml.
Figure 2: Percentage of inhibition of human erythrocyte membrane destabilization.
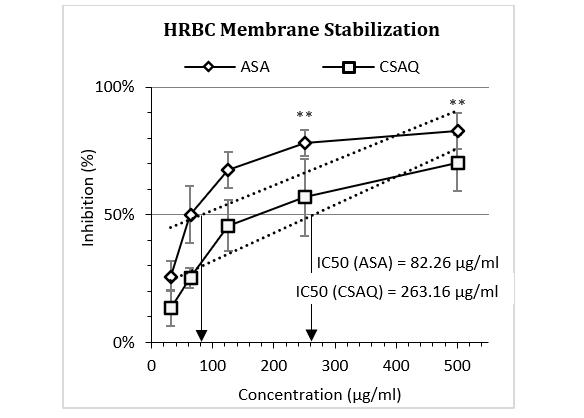
Data represented as mean (%) ± SEM, (n = 5); IC50 calculated from linear regression; **p < 0.01; Sample T-test treated one group as control and compared all other groups against it. X-axis, applied concentrations were 31.25; 62.5, 125, 250 and 500 µg/ml.
Temperature Induced Haemolysis Assay
Figure 3 (a-b) articulated the percentage inhibition of haemolysis in different thermal conditions – at excess heat and at loss of heat. Both ASA and CSAQ reached to their IC50 in higher concentrations (272.42 µg/ml and 318.27 µg/ml respectively) at the hyperthermic condition (Fig. 3a). On the contrary, in cold temperature IC50 was achieved by the two groups at 247.85 µg/ml and 288.25 µg/ml respectively. In addition, the inhibition of haemolysis was found higher in percentage in hypothermic condition in all concentrations for both groups. For example, Aspirin inhibited 75.49% in heat and 79.55% in cold conditions, at 500 µg/ml concentration.
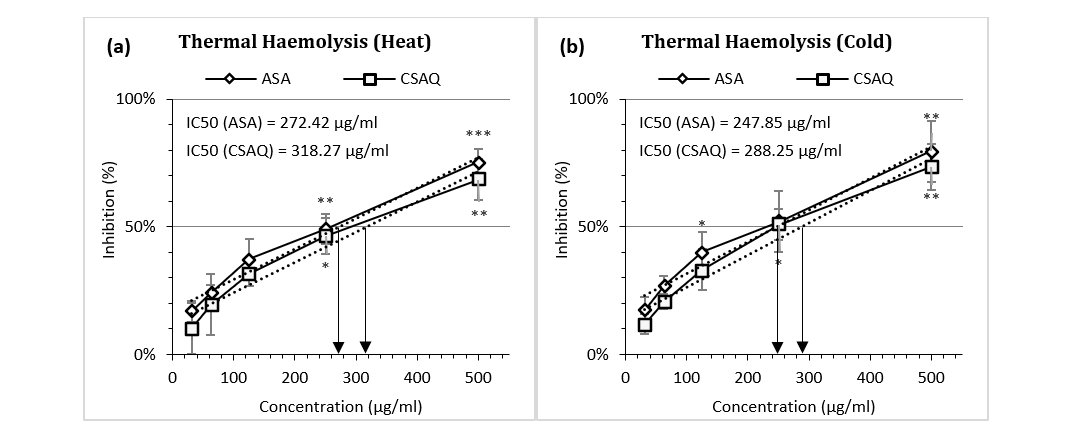
Figure 3 (a-b): Percentage of inhibition of thermal heamolysis (a) in heat (b) in cold.
Data represented as mean (%) ± SEM, (n = 5); IC50 calculated from linear regression; *p < 0.05, **p < 0.01, ***p < 0.001; Sample T-test treated one group as control and compared all other groups against it. X-axis, applied concentrations were 31.25; 62.5, 125, 250 and 500 µg/ml.
Tonicity Induced Haemolysis Assay
Like thermal variation, percentage inhibition of haemolysis was also observed in various environmental conditions – in hypotonic (Figure 4a) and isotonic solutions (Figure 4b). IC50 for aspirin was found at 317.43 µg/ml in hypotonic solution and 242.89 µg/ml in isotonic solution whereas, IC50 for sample was achieved in 358.72 µg/ml and 289.89 µg/ml respectively. For both ASA and CSAQ, RBC lysis was found less in percentages in isotonic solution than in hypotonic solution.
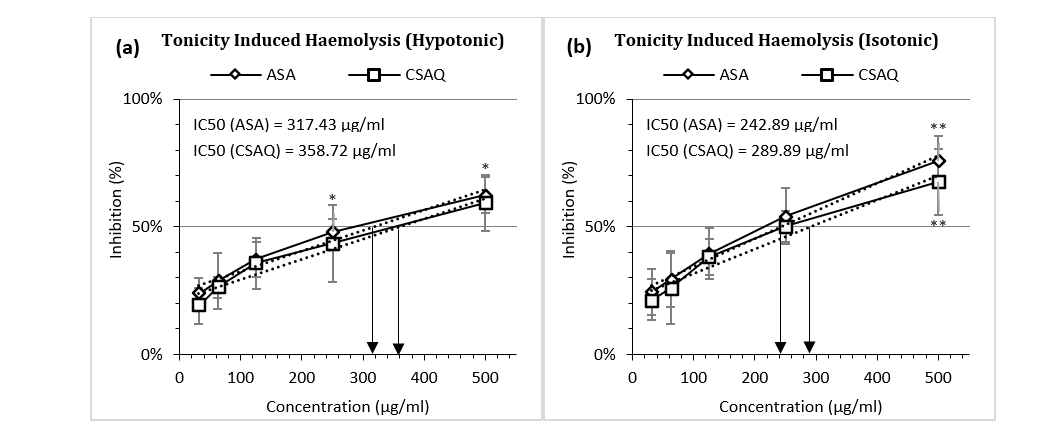
Figure 4 (a-b): Percentage of inhibition of heamolysis (a) in hypotonic solution (b) in isotonic solution.
Data represented as mean (%) ± SEM, (n = 5); IC50 calculated from linear regression; *p < 0.05, **p < 0.01; Sample T-test treated one group as control and compared all other groups against it. X-axis, applied concentrations were 31.25; 62.5, 125, 250 and 500 µg/ml.
Discussion
Inflammation involves a series of cellular changes including destabilization of lysosomal membrane and denaturation of proteins. Thus, to model the in vitro inflammation, the methods of egg protein denaturation assay, HRBC membrane stabilization assay, heat and hypotonicity-induced haemolysis assays were adopted in this study. The protective ability of C. sinensis fruit peel was assessed and compared with the standard Acetyl Salicylic Acid (Aspirin).
Protein denaturation produces a chronic inflammatory response through a biochemical reaction to attenuate the tissue functions (25,26). When lysosomal membranes are disrupted, pro-inflammatory markers are released. These markers activate mediators like neutrophils, proteases and histamines at the site of disruption (27). The phenomenon causes type III hypersensitivity reactions associated with diseases like glomerulonephritis and serum sickness (24). Conventional NSAIDs like Acetyl Salicylic Acid suppress COX enzyme and endogenous PGE2 synthesis, however, also reported to bock the protein denaturation process (28). Aspirin, in this study increased the percentage of inhibition of protein denaturation with increasing concentrations. C. sinensis peel extract mimicked the standard though resulted in lower inhibitory potential in comparison.
Erythrocyte membrane destabilization is the consequence of heat induced hemolysis as it promotes the production of free radicals like lipid peroxides and superoxides (29). The membrane is similar to the lysosomal membrane (30). The rupture of the membrane makes the RBC more prone to secondary damages. According to the mechanism of actions, most anti-inflammatory drugs either block the release of lysosomal enzymes or stabilize the lysosomal membrane in order to suppress the inflammatory processes (22). Both Aspirin and the extract showed a dose-dependent increase in exhibiting the protective role against RBC lysis.
The protective effects of the extract and the standard were assessed again with HRBC in different environmental conditions (i.e., with elevated heat to cold temperature and with hypotonic and isotonic solutions). Higher temperature potentiates increase in cell membrane permeability and facilitates leakage of cellular fluids into outer space making the cell more vulnerable to damage (31). On the other hand, hypotonic solution is by nature hemolytic as it causes accumulation of excessive fluid into the cells which results in disruption of RBC membrane (24). As opposite, isotonic solution facilitated with a much favorable environment for membrane stabilization. The outflow of serum protein and cellular fluids into the tissue compartment are prevented by membrane stabilization (32). As a consequence, the sample and the standard demonstrated better efficacy in isotonic and cold environment.
Phytochemical analysis of the extract illustrated a composition of protective components like tannin, phenol, flavonoid, reducing sugar, protein, carbohydrate and steroid. This was in accordance with other studies which confirmed the presence of terpenoids, alkaloids, steroids, glycosides and flavonoids in the peel extracts (33). Physicochemical screening indicated to its highly stable compound composition (20). Altogether, the extract possesses high potential against free radicals which in turn favors anti-inflammatory actions and the obtained results can be attributed to its nature and content.
Conclusion
Findings suggest that C. sinensis peel carries a significant potential as a natural anti-inflammatory agent. It showed a concentration-dependent increase in protecting the egg protein and red blood cell membrane. Moreover, the results were closely comparable to that of the standard. This potentiates further investigation for a complete screening of phytochemical composition to identify the responsible bioactive compounds.
Abbreviations
HRBC: Human Red Blood Cell; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; ASA: Acetyl Salicylic Acid; CSAQ: C. sinensis fruit peel aqueous extract; COX: Cyclooxygenase; PG: Prostaglandin; OD: Optical Density; IC: Inhibitory Concentration.
Acknowledgments
The present study was supported and carried out in the Pharmacology lab of Institute for Pharmaceutical Skill Development and Research, Bangladesh. Authors are grateful to the institution for providing such opportunity to contribute to health science.
Authors’ Contributions
This work was carried out in collaboration between
all authors. Authors KN and MMB designed coordinated and supervised the project. KN and FA performed in vitro experiments and prepared the graphical presentations. RRT participated in the assessment of physicochemical and phytochemical properties and prepared the manuscript. AH participated in the experiments and critically revised the manuscript. MNI participated in interpretation of data to reach a scientific discussion. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was carried out with individual funding of all authors.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All experiments associated with human sampling were performed in accordance to the Helsinki Declaration, adopted in the institutional ethical committee guideline (Ref. no. IPSDRLAB/EHSP/ 01/18). Volunteers were selected prior taking consent in written Informed Consent Forms (Ref. No. IPSDRLAB/ICF/23/20) explaining the purpose of the investigation.
The experimental design was authorized by the Institutional Ethical Committee Clearance (Ref. No. IPSDRLAB/IECC/23/20) from the Institute for Pharmaceutical Skill Development and Research, Bangladesh (Project approved on 26/02/2020).
Consent for Publication
Not applicable.
Competing Interests
All authors agreed on the article before submission and had no conflict of interests.
References
- Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204-7218. DOI PMC Google Scholar Link
- DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30(11):547-556. DOI PMC Google Scholar Link
- 3. Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of disease, professional edition e-book. Elsevier health sciences; 2014 Aug 27. Accessed June 7, 2021. Google Scholar Link
- Maldini M, Sosa S, Montoro P, et al. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Hitchcock. J Ethnopharmacol. 2009;122(3):430-433. DOI PubMed Google Scholar Link
- Famitafreshi H, Karimian M. Prostaglandins as the Agents That Modulate the Course of Brain Disorders. Degener Neurol Neuromuscul Dis. 2020;10:1-13. DOI PubMed Google Scholar Link
- Goldstein JL, Cryer B. Gastrointestinal injury associated with NSAID use: a case study and review of risk factors and preventative strategies. Drug Healthc Patient Saf. 2015;7:31-41. DOI PMC Google Scholar Link
- Wongrakpanich Supakanya WA, Wongrakpanich Supakanya WA. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging and disease. 2018;9(1):143-150. DOI PMC Google Scholar Link
- McCarberg B, Gibofsky A. Need to Develop New Nonsteroidal Anti-Inflammatory Drug Formula-tions. Clinical Therapeutics. 2012;34(9):1954-1963. DOI PubMed Google Scholar Link
- Denaro M, Smeriglio A, Trombetta D. Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion. Antioxidants. 2021;10(2):140. DOI PubMed Google Scholar Link
- Khan RA, Mallick N, Feroz Z. Anti-inflammatory effects of Citrus sinensis L., Citrus paradisi L. and their combinations. Pak J Pharm Sci. 2016;29(3):843-852. PubMed Google Scholar Link
- Nasri M, Bedjou F, Porras D, Martínez-Flórez S. Antioxidant, anti-inflammatory, and analgesic activities of Citrus reticulata Blanco leaves extracts: An in vivo and in vitro study. Phytothérapie. Published online January 24, 2017. DOI Google Scholar Link
- Plastina P, Apriantini A, Meijerink J, Witkamp R, Gabriele B, Fazio A. In Vitro Anti-Inflammatory and Radical Scavenging Properties of Chinotto (Citrus myrtifolia Raf.) Essential Oils. Nutrients. 2018;10(6):783. DOI PubMed Google Scholar Link
- Leguizamón NDP, Rodrigues EM, de Campos ML, et al. In vivo and in vitro anti-inflammatory and pro-osteogenic effects of citrus cystatin CsinCPI-2. Cytokine. 2019;123:154760. DOI PubMed Google Scholar Link
- Huang Y-S, Ho S-C. Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel. Food Chemistry. 2010;119(3): 868-873. DOI Google Scholar Link
- Oikeh EI, Oviasogie FE, Omoregie ES. Quantitative phytochemical analysis and antimicrobial activities of fresh and dry ethanol extracts of Citrus sinensis (L.) Osbeck (sweet Orange) peels. Clin Phytosci. 2020;6(1):46. DOI Google Scholar Link
- Kanaze FI, Termentzi A, Gabrieli C, Niopas I, Georgarakis M, Kokkalou E. The phytochemical analysis and antioxidant activity assessment of orange peel (Citrus sinensis) cultivated in Greece-Crete indicates a new commercial source of hesperidin. Biomed Chromatogr. 2009;23(3): 239-249. DOI PubMed Google Scholar Link
- Parmar HS, Kar A. Medicinal values of fruit peels from Citrus sinensis, Punica granatum, and Musa paradisiaca with respect to alterations in tissue lipid peroxidation and serum concentration of glucose, insulin, and thyroid hormones. J Med Food. 2008;11(2):376-381. DOI PubMed Google Scholar Link
- Selmi S, Rtibi K, Grami D, Sebai H, Marzouki L. Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat. Lipids in Health and Disease. 2017;16(1):152. DOI PMC Google Scholar Link
- Giri B. Phytochemical Screening, Antioxidant, Antibacterial Activities of Citrus limon and Citrus Sinensis Peel Extracts. IPCM. 2017;1(2). DOI Link
- Rayhan MA, Vabna NJ, Ahmed F, Hossin A, Nawrin K, Billah MM. Jujube (Ziziphus jujube) honey treats stress induced anxiety behavior in mice. Pharmacotherapy and Pharmascience Discovery. 2021;1(1):1-9. DOI Google Scholar Link
- Obiang CS, Misso RLNM, Atome GRN, et al. Antimicrobial, antioxidant, anti-inflammatory and cytotoxic study of extracts of Guibourtia tessmanii (harms) J. Léonard from Gabon. Clinical Phytoscience. 2021;7(1):45. DOI Google Scholar Link
- Malleshappa P, Kumaran R, Venkatarangaiah K, Parveen S. Peels of Citrus Fruits: A Potential Source of Anti-inflammatory and Anti-nociceptive Agents. Pharmacognosy Journal. 2018;10(6s):s172-s178. DOI Google Scholar Link
- Kumar K, Manickam S, Sreejith M, Sebastin V. In Vitro and In Vivo Anti-Inflammatory Evaluation of the Whole Plant Extracts of Crotalaria Biflora (L). Pharmacognosy Journal. 2021;13(3):620-625. DOI Google Scholar Link
- Yesmin S, Paul A, Naz T, et al. Membrane stabilization as a mechanism of the anti-inflammatory activity of ethanolic root extract of Choi (Piper chaba). Clinical Phytoscience. 2020; 6(1):59. DOI Google Scholar Link
- 25. Sangeetha, G., Vidhya R. “In vitro anti-inflammatory activity of different parts of Pedalium murex (L.).” International Journal of Herbal Medicine 4 (2016): 31-36. Google Scholar Link
- Ikwegbue PC, Masamba P, Oyinloye BE, Kappo AP. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals (Basel). 2017;11(1). DOI PMC Google Scholar Link
- Anyasor GN, Okanlawon AA, Ogunbiyi B. Evaluation of anti-inflammatory activity of Justicia secunda Vahl leaf extract using in vitro and in vivo inflammation models. Clinical Phytoscience. 2019;5(1):49. DOI Google Scholar Link
- Ranasinghe P, Ranasinghe P, Abeysekera WPKM, et al. In vitro erythrocyte membrane stabilization properties of Carica papaya L. leaf extracts. Pharmacognosy Res. 2012;4(4):196-202. DOI PubMed Google Scholar Link
- Richards DMC, Dean RT, Jessup W. Membrane proteins are critical targets in free radical mediated cytolysis. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1988;946(2):281-288. DOI PubMed Google Scholar Link
- Shenoy S, Shwetha K, Prabhu K, Maradi R, Bairy K, Shanbhag T. Evaluation of antiinflammatory activity of Tephrosia purpurea in rats. Asian Pacific Journal of Tropical Medicine. 2010;3(3): 193-195 DOI Google Scholar Link
- Saso L, Valentini G, Casini ML, et al. Inhibition of heat-induced denaturation of albumin by nonsteroidal antiinflammatory drugs (NSAIDs): pharmacological implications. Arch Pharm Res. 2001;24(2):150-158. DOI PubMed Google Scholar Link
- Singh B, Sahu PM, Sharma MK. Anti-inflammatory and antimicrobial activities of triterpenoids from Strobilanthes callosus Nees. Phytomedicine. 2002;9(4):355-359. DOI PubMed Link
- Mehmood B, Dar KK, Ali S, et al. Short communi-cation: in vitro assessment of antioxidant, antibacterial and phytochemical analysis of peel of Citrus sinensis. Pak J Pharm Sci. 2015;28(1):231-239. PubMed Google Scholar Link
Update History
| Revision Number | Date | Details Of Changes |
|---|---|---|
| 01 | 2021-06-13 | Original Article; published at its accepted version (Reference Number: PPD/MIN/2161A) |
 Pharmacotherapy & Pharmascience Discovery
Pharmacotherapy & Pharmascience Discovery
