- Received On: 2021-07-03|
- Accepted On: 2021-10-22|
- Published On: 2021-11-11
| Article Metadata | |||
|---|---|---|---|
| 1 | Submitted Manuscript | PPD/MIN/217/1 | |
| 2 | Cover Letter to Editor | PPD/CLE/217/1 | |
| 3 | Copyright Transfer Letter | PPD/CTL/217/1 | |
| 4 | Authors’ Consent Letter | PPD/ACL/217/1 | |
| 5 | Initial Editorial Screening Report | PPD/IESR/217/1 | |
| 6 | Review Agreement Letter (Reviewer 1) | PPD/RAL/2171/R1 | |
| 7 | Review Agreement Letter (Reviewer 2) | PPD/RAL/2171/R2 | |
| 8 | Manuscript Review Report (Round 1, Reviewer 1) | PPD/MRR/2171/R1.1 | |
| 9 | Manuscript Review Report (Round 1, Reviewer 2) | PPD/MRR/2171/R1.2 | |
| 10 | Revised Manuscript | PPD/MIN/2171R | |
| 11 | Review Response Letter (Round 1) | PPD/RRL/2171/R1 | |
| 12 | Manuscript Review Report (Round 2, Reviewer 1) | PPD/MRR/2171/R2.1 | |
| 13 | Manuscript Review Report (Round 2, Reviewer 2) | PPD/MRR/2171/R2.2 | |
| 14 | Final Editorial Screening Report | PPD/FESR/2171 | |
| 15 | Letter of Acceptance and Acknowledgement | PPD/LAA/2171 | |
| 16 | Accepted Manuscript | PPD/MIN/2171A | |
| 17 | Galley Proof Manuscript | PPD/MIN/GPM/2171 | |
| Request Access | |||
| Supplementary Data | ||
|---|---|---|
| The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request. | ||
| Datasets | Not Provided | Request Availability |
Abstract
Background: The leaf and root of O. sanctum has long been among the primary choices in herbal treatment of cough. The present study was aimed to formulate an optimized mixture of leaf and root extracts and assess their efficacy as cough suppressant and expectorant individually and in combination with the standards.
Methods: Sulphur Dioxide (SO2)- and capsaicin-induced cough model was developed to assess antitussive activity whereas broncho-tracheal phenol red secretion model was developed to evaluate expectorant activity in mice. Individual application of aqueous leaf and root extract (100, 200 & 400 mg/kg) was compared with standards. Moreover, the formulated leaf to root extract (50:150, 100:100; 150:50 mg/kg) was brought in to comparison. Finally, all extract dose groups were conjugated with one-tenth dose of the standards and intra-comparison between all groups were performed.
Results: Data showed that OSL 400 and OSR 400 both inhibited the cough reflexes though OSR activity was not with the mark of OSL. The activity pattern was found similar in formulated mixture too where OSL:R 150:50 dominated. When coupled with standards, the extract mixture demonstrated greater efficacy in comparison to that of individual extracts. Interestingly, the mixture conjugated with the standard’s one-tenth dose (1 mg/kg), proved equivalently potent as standard at its individual dose (10 mg/kg).
Conclusion: The findings suggested an insight for optimized mixture of leaf and root of O. sanctum, which can suppress and/or expel cough, coupling with low dose of standards those are associated with side effects.
Introduction
Medical science describes Cough as a defensive reflex of the respiratory tract and is considered favorable to human body to clear the upper airways unless it becomes aggravated and interfere with the normal respiration [1,2]. Dry or unproductive cough are often linked with eosinophilic bronchitis, airways irritation as a result of air pollutants, airways allergies, gastroesophageal reflux disease (GERD) [2,3], however, sometimes occurs without any associated root, often termed as idiopathic cough [4]. Though steam inhalation, use of demulcents are found quite effective in most of the events through hydration of respiratory tract, uncontrolled coughs are treated with opioidergic central cough suppressants [5]. Codeine, pholcodeine, noscapine, dextromethorphan is among the frequently prescribed opioids, however, certainly comes with associated side effects like sedation, addiction, nausea, apnea, constipation etc [6,7]. Moreover, use of these drugs are contraindicated in severe respiratory congestion like asthma as they are known to compromise the respiratory activities further [8]. Rationally, the search for effective as well as safe antitussive agents has become a prime need in field of respiratory phytomedicine.
Many plants have been in claim for anti-asthmatic or cough suppressive properties. Extensive scientific studies have been performed based on the recommendations from the folk medical practitioners and tribes. Among these, a most widely used antitussive plant is Ocimum sanctum Linn. (Lamiaceae), also known as Holy Basil, and considered a herb for all reasons [9-11]. The leaf of the plant is considered both a cure and prevention for cough and cold [12-14]. Alongside, the root of this plant has been reported to have expectorant and antitussive activities [9,15]. The design of this study thereby started with the aim to evaluate antitussive and expectorant properties of both leaf and root of O. sanctum, further compare their effectiveness in combination and finally assess for synergies when used together with standards.
Methods
Collection and Preparation of the Extracts
Total 10 O. sanctum whole plants were collected from kalatiya, keraniganj, Dhaka (23°42'59"N, 90°17'8"E) and authenticated from Bangladesh National Herbarium and a specimen was kept with an accession number 56349. The plants were washed thoroughly with running water and leaves and roots were isolated. Both the plants parts were then subjected to dry under ceiling fan and afterwards, crushed. In powder form, leaves and roots were soaked in distilled water separately and left for 3 days with occasional shaking. The mixtures were filtered with paper filters and the filtrates were concentrated using a rotary evaporator (Biobase RE-2010, China) [16]. Finally, approximately 5g of leaf (OSL) and 3g of root (OSR) extracts were obtained.
Drugs and Chemicals
Codeine phosphate (Merck, Germany), capsaicin (Incepta Pharmaceuticals Ltd., Bangladesh), salbutamol (Square Pharmaceuticals Ltd.), phenol red (Sigma-Aldrich, USA) and sodium hydrogen sulfite and sulphuric acid (RCI LABSCAN Limited, Thailand), sodium hydroxide (Merck, Germany) were obtained. All reagents were of analytical grades.
Experimental Animals
All experiments were conducted on Swiss Albino mice of both sexes, weighing 26-32g and aged between 45-52 days. Animals were purchased from the animal house of pharmacy department of Jahangirnagar University and kept in semi-transparent plastic cages with adequate food and water supply. They are fed with standard dried pellets and allowed a 12h light and dark cycle.
Acute Toxicity Test
Oral gavage was performed with high doses (250, 500, 1000 and 2000 mg/kg) of the plant extract to five healthy mice comprising each group to investigate the immediate and short-term toxicity. They were monitored for the next 3 days for unusual behavior or any mortality [17].
Grouping of Animal
Prior experiments, 120 mice were divided into 20 groups each consisting of 6 mice. Groups were designated with respective agents as follows: Group 1: Control, received distilled water. Group 2: Positive Control, received standard drug. Group 3: OSL 100 mg/kg. Group 4: OSL 200 mg/kg. Group 5: OSL 400 mg/kg. Group 6: OSR 100 mg/kg. Group 7: OSR 200 mg/kg. Group 8: OSR 400 mg/kg. Group 9: OSL:R 50:150 mg/kg. Group 10: OSL:R 100:100 mg/kg. Group 11: OSL:R 150:50 mg/kg. Group 12: OSL 100 mg/kg + Standard. Group 13: OSL 200 mg/kg + Standard. Group 14: OSL 400 mg/kg + Standard. Group 15: OSR 100 mg/kg + Standard. Group 16: OSR 200 mg/kg + Standard. Group 17: OSR 400 mg/kg + Standard. Group 18: OSL:R 50:150 mg/kg + Standard. Group 19: OSL:R 100:100 mg/kg + Standard. Group 20: OSL:R 150:50 mg/kg + Standard. All groups received the treatments orally.
Assessment of Antitussive Properties
Sulphur Dioxide (SO2)-Induced Cough Model
Antitussive activity was assessed according to the method described by Miyagoshi et al., 1986 [18] and simplified by Gupta et al., 2009 [2]. A desiccator containing a vial (2ml) at the base, holding a pipette from the top and floored with a wired gauge, was used in this experiment. At first, the vial contained 500mg/ml sodium hydrogen sulfite (NaHSO3) and 0.2 ml of sulphuric acid (H2SO4) was added using the top mounted pipette where the following reaction took place:
2NaHSO3 + H2SO4 = 2SO2 + Na2SO4 + H2O
Mice were placed in the wired platform in the desiccator after 15 seconds and subjected to the exposure of SO2 for 45 seconds. Thereafter, mice were withdrawn from the desiccator and placed in observation cage with an open-ended funnel filter to which a stethoscope was attached, to count the reflexes of cough for 5 minutes. The experiment was repeated for all groups at 30, 60, 90 and 120 minutes after oral drug treatments [19].
Capsaicin-Induced Cough Model
This experiment was performed according to the model described by Zhang et al., 2009 [20] and modified by W. Liu et al., 2015 [21]. A 500 ml glass beaker was used in this regard where the mice were individually placed and subjected to atomized spray of capsaicin solution (100μmol/l) for 10 seconds. The frequency of coughing was counted for 2 minutes. After recovery on 24h, mice were orally administered with the respective treatments and re-exposed to capsaicin nebulization. Again, the bouts of coughing were recorded.
Assessment of Expectorant properties
Phenol Red Secretion Model
The method was carried out as described by Han et al., 2010 [22]. 5% phenol red physiological saline (500mg/kg) was intraperitoneally injected to mice after 30 minutes of drug and test agents’ administration. After another 30 minutes, mice were sacrificed keeping the trachea intact from the thyroid cartilage to the main stem bronchi. This part was removed and sonicated with 1.5 ml of physiological saline solution to extract phenol red. 100 µl of the extraction was transferred to a 96-wells plate and kept in 100 µl of 0.1M sodium hydroxide. The optical density was immediately measured with a microplate reader (Biobase-EL10A, China) at 546nm. The amount of phenol red was deduced from the regression curve of different concentration.
Statistical Analysis
Data represented as mean ± standard error (n=5). To assess the difference between the observations before and after treatment, paired t test was conducted. To determine the statistical significance one way analysis of variance (ANOVA) was performed using SPSS v.20. p values were assessed within range of 0.001 < p < 0.05 and a value less than 0.05 was considered as statistically significant.
Results
No abnormal behavioral symptoms or mortality was recorded during treatment or in its follow-up. However, mild to moderate indigestions characterized by greenish yellow stool were recorded at higher doses like 1000 and 2000 mg/kg. Rationally, lower doses (100, 200, 400 mg/kg body weight of mice) were used for further experiments.
On treatment of O. sanctum leaf extract against sulphur dioxide (SO2) induced cough, OSL 400 was found significantly effective in inhibiting the bouts of cough (Figure 1a). More profound efficacy was observed when the same dose was applied in combination with codeine phosphate at 1 mg/kg (18.4, 60 min). Codeine alone at 10 mg/kg showed a gradual reduction in the frequency of cough till 90 minutes (20.7).
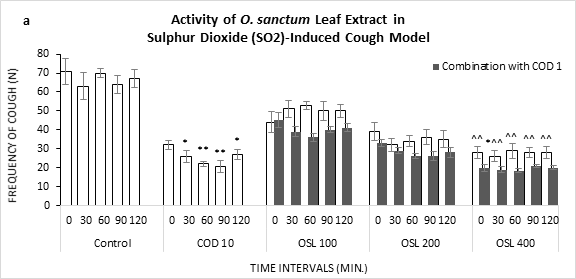
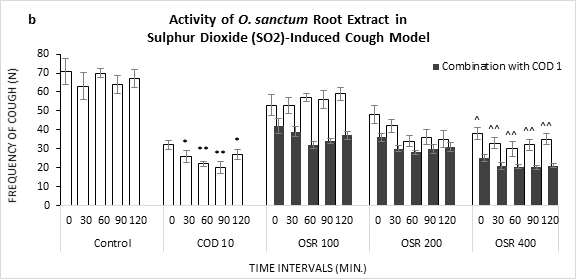
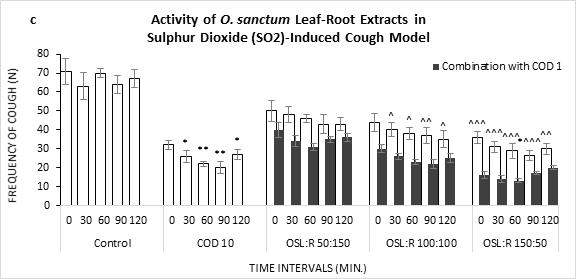
Figure 1: Frequency of Cough observed in Sulphur Dioxide (SO2) induced cough model against the treatment of O. sanctum (a) leaf extract (OSL), (b) root extract (OSR), (c) leaf to root extract in ratio (OSL:R), measured over time. COD=Codeine Phosphate. Data represents as mean ± standard error mean (SEM) (n=6) against the concentrations in mg/kg. To compare all groups against control, Dunnett t test was performed alongside one way analysis of variation (ANOVA). *&^ represented p value for individual groups and combination groups respectively; */^, **/^^, ***/^^^ denoted p<0.05, 0.01, 0.001 respectively and considered statistically significant.
Unlike leaf extract, O. sanctum root extract showed lesser potential to decrease coughing frequencies (Figure 1b). In comparison to control, OSR 100 could not limit the bouts alone. However, along with the standard (1mg/kg), OSR 100 reduced the number of coughs. Maximum efficacy for the root extract and codeine combination was found with OSR 400 at 60 min (20.1).
Formulated extract of leaf and root exhibited a linear fall in frequencies of cough over time in all ratio (Figure 1c). Treatment with leaf dominant formulation showed greater efficacies (26.2, 90 min) in comparison to root dominant and equal formulation. Moreover, the conjugation of codeine accelerated the inhibition, maximum at 60 min (13.0).
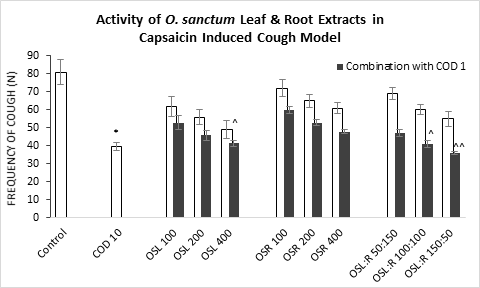
Figure 2: Frequency of Cough observed in capsaicin induced cough model against the treatment of O. sanctum (a) leaf extract (OSL), (b) root extract (OSR), (c) leaf to root extract in ratio (OSL:R), measured in 2 minutes after drug/test agent administration. COD=Codeine Phosphate. Data represents as mean ± standard error mean (SEM) (n=6) against the concentrations in mg/kg. To compare all groups against control, Dunnett t test was performed alongside one way analysis of variation (ANOVA). *&^ represented p value for individual groups and combination groups respectively; */^, **/^^, ***/^^^ denoted p<0.05, 0.01, 0.001 respectively and considered statistically significant.
Like Sulphur Dioxide (SO2) induced cough test, mice undergo less cough when treated with the standard (39.5) (Figure 2) in capsaicin-Induced Cough Model. On leaf extract treatment, OSL 400 alone significantly treated the cough (41.4), however, lower doses proved to be less potent. More prominent potential was shown by the leaf dominant formulated extract OSL:R 150:50 in combination with codeine phosphate at 1mg/kg (36.1).
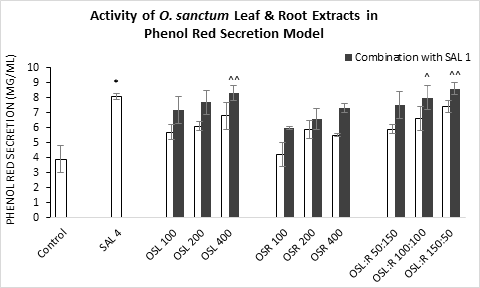
Figure 3: Secretion of Phenol Red observed in mice trachea against the treatment of O. sanctum (a) leaf extract (OSL), (b) root extract (OSR), (c) leaf to root extract in ratio (OSL:R). SAL=Salbutamol. Data represents as mean ± standard error mean (SEM) (n=6) against the concentrations in mg/kg. To compare all groups against control, Dunnett t test was performed alongside one way analysis of variation (ANOVA). *&^ represented p value for individual groups and combination groups respectively; */^, **/^^, ***/^^^ denoted p<0.05, 0.01, 0.001 respectively and considered statistically significant.
Maximum phenol red secretion was observed by the Salbutamol at 4 mg/kg body weight (8.1) in comparison to the control group (3.9 mg/ml) (Figure 3). OSL 400 and OSL:R 150:50, both in conjugation with standard at 1 mg/kg, greatly increased the secretion level (8.3 and 8.6 mg/ml respectively). the extract in their individual application showed moderate increase in phenol red secretion.
Discussion
As a traditional folk medicine, the leaf and root of O. sanctum were explored against cough in sulphur dioxide (SO2)- and capsaicin-stimulated mice models. Inhalation of high concentrations of SO2 causes irritation and pulmonary and systemic inflammation, thus, can affect lung function and worsen asthma attacks [23,24]. Acute symptoms of SO2 exposure include wheezing, shortness of breath and chest tightness [25]. Early response of SO2 inhalation involves tissue injury, neutrophilic lung inflammation and airway hyperresponsiveness (AHR) [26]. SO2 results in lipid peroxidation and oxidative damage in lungs of mice. causing a significant increase of TBARS and a significant decrease in GSH content in lungs, significantly increased SOD and GPx activities, however, reported to decrease CAT activities [27].
On the other hand, inhalation of capsaicin nebulized spray causes central respiratory depression and fatal apneas mediated via TRPV1 activation on lung afferents, spinal cord-ascending tracts, and medullary structures (including nucleus tractus solitarius). AMPA receptor-triggered conductances act a vital role in capsaicin-induced apneas [28]. Thus, the drug development can be targeted at reducing AMPA receptor-mediated glutamatergic signaling. The opioid codeine phosphate has been in the core of the cough treatment for long and thus regarded as the 'gold standard' cough suppressant. Codeine restricts the tracheal constriction along with cough reflex in addition to its central cough suppressant action [29].
The increase in the level of tracheal phenol red links to an increased hexose concentration in the lung fluid. This indicates a triggered mucin secretion from mucous cells of submucosal glands and goblet cells of the surface epithelium in airways [30]. The intratracheal secretion of phenol red is also significantly increased by both parasympathomimetics and sympathomimetics. Along-side, an increase in mucociliary clearance may facilitate the transport of more phenol red into the trachea and thus augments phenol red concentrations [31]. Expectorants are found to increase the secretion of mucins and/or mucus hydration to an extent which produce a sufficient mass of mucus to be coughed up or sneeze to expel the mucus from the lungs or upper respiratory tract [32-35]. This may function as irritants to gastric vagal receptors, and recruit efferent parasympathetic reflexes inducing glandular exocytosis of a diluted mucus mixture [36,37].
Salbutamol is a recognized β2-adrenergic agonist that facilitates mucin secretion and at 4 mg/kg interaperitoneal administration, enables phenol red detection in trachea [31]. Moreover, being a β2 adrenoceptor agonists salbutamol exerts its maximum therapeutic potential through bronchodilation, tempting in the respiratory smooth muscle synthesis of the cyclic adenosine monophosphate (cAMP) pathway. cAMP is a molecule with various cellular functions. An increase in the cAMP-dependent protein kinase A activity facilitates smooth muscle relaxation resulting in bronchodilation [38]. Furthermore, report confirmed that inhalation of salbutamol rises mucociliary clearance rate, a crucial defense mechanism for clearing the unwanted mass from lung in subjects with asthma and chronic bronchitis, via increase in ciliary beat frequency shown in vitro and in vivo studies [39-41].
From the observation, it can be concluded with four noticeable findings which follows as firstly, both root and leaf extract of O. sanctum was prominent in showing their antitussive and expectorant activities alongside the standards. Secondly, leaf was profound in efficacy in comparison to root extract and the same case was observed in leaf dominant leaf-root formulated extract. Thirdly, when conjugated with the standard, extracts showed synergies in efficacy and finally, application of the extracts in conjugation with one-tenth (1 mg/kg) of the dose of the standard as applied in sole treatment (10 mg/kg) generated equivalent outputs, thus, indicated a scope for minimizing the dose of drugs associated with side effects.
The chemical composition of O. sanctum is highly complex, where the leaves and stem contain biologically active constituents including saponins, flavonoids, triterpenoids, and tannins, though, varies largely within strains and location [42]. The leaf volatile oil is constituted of eugenol, euginal (eugenic acid), urosolic acid, carvacrol, linalool, limatrol, caryophyllene, methyl carvicol (Estragol) [43,44]. In addition, rosmarinic acid, propanoic acid, apigenin, cirsimaritin, isothymusin and isothymonin rosmarinic acid, propanoic acid, apigenin, cirsimaritin, isothymusin and isothymonin also exhibit antioxidant and antiinflammatory activities [9]. The obtained findings were thus assumed to be attributed to these compounds and together with the establish mechanism of the standards, where applied in drug-extract combination. However, at this point of study it was not possible to pin point the responsible biological active compounds for certain cough suppressant and expectorant activities. Therefore, fractionization of the extract along with the phytochemical screening was recommended to undergo further in vivo assessment.
Conclusion
O. sanctum is in the mainstay of the treatment of cough for folk medicine prescribers. This study supported the scientific proof for the basis of its use and also provided an insight to draw optimized dose line and a possibility to reduce the dose of standard drugs associated with side effects. From the findings, it can be confirmed that, both leaf and root of O. sanctum possess cough suppressant and expectorant activities.
Abbreviations
TBARS: Thiobarbituric Acid Reactive Substances; GSH: Glutathione; SOD: Superoxide Dismutases; GPx: Glutathione Peroxidase; CAT: Catalase; TRPV1: Transient Receptor Potential Vanilloid-1; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; cAMP: Cyclic Adenosine Monophosphate; OSL: O. sanctum Leaf; OSR: O. sanctum Root; OSL:R: O. sanctum leaf to root extract in ratio; SO2: Sulphur Dioxide.
Acknowledgments
The present study was supported and carried out in the Pharmacology lab of Institute for Pharmaceutical Skill Development and Research, Bangladesh.
Authors’ Contributions
This work was carried out in collaboration between all authors. Authors KN and MMB designed coordinated and supervised the project. MMB, MSUJ, KN, AH performed in vitro experiments. MMB and KN prepared the graphical presentations. AH prepared the manuscript. KN critically revised the manuscript. MNI analyzed and interpreted the data to reach a scientific discussion. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was carried out with individual funding of all authors.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All experiments associated with animal handling were performed in accordance with the Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection adopted by the institutional guideline for animal handling (Ref. no. IPSDRLAB/AHCP/ 01/18). The experimental design was authorized by the Institutional Ethical Committee Clearance (Ref. No. IPSDRLAB/IECC/18/19) from the Institute for Pharmaceutical Skill Development and Research, Bangladesh.
Consent for Publication
Not applicable.
Competing Interests
All authors agreed on the article before submission and had no conflict of interests.
References
- Polverino M, Polverino F, Fasolino M, Andò F, Alfieri A, De Blasio F. Anatomy and neuro-pathophysiology of the cough reflex arc. Multidiscip Respir Med. 2012;7(1):5. DOI PubMed PMC Google Scholar Link
- Gupta YK, Katyal J, Kumar G, et al. Evaluation of antitussive activity of formulations with herbal extracts in sulphur dioxide (SO2) induced cough model in mice. Indian J Physiol Pharmacol. 2009;53(1):61-66. PubMed Google Scholar Link
- Lai K, Pan J, Chen R, Liu B, Luo W, Zhong N. Epidemiology of cough in relation to China. Cough. 2013;9(1):18. DOI PubMed PMC Google Scholar Link
- Chung KF. Approach to chronic cough: the neuropathic basis for cough hypersensitivity syndrome. J Thorac Dis. 2014;6(Suppl 7):S699-S707. DOI PubMed PMC Google Scholar Link
- Paneliya DAM. Antitussive activity of Vasa Avaleha formulations on sulfur dioxide‐induced coughing in mice. International Journal of Green Pharmacy (IJGP). 2015;9(3):180-183. DOI Google Scholar Link
- Eddy NB, Friebel H, Hahn KJ, Halbach H. Codeine and its alternates for pain and cough relief. Bull World Health Organ. 1969;40(3):425-454. PubMed PMC Google Scholar Link
- Randerath WJ, George S. Opioid-Induced Sleep Apnea: Is It a Real Problem? J Clin Sleep Med. 2012;8(5):577-578. DOI PubMed PMC Google Scholar Link
- Promethazine HCl and Codeine Phosphate Oral Solution. [online] Accessdata.fda.gov. 2017. Accessed November 11, 2021. Link
- Pattanayak P, Behera P, Das D, Panda SK. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacogn Rev. 2010;4(7):95-105. DOI PubMed PMC Google Scholar Link
- Cohen MM. Tulsi - Ocimum sanctum: A herb for all reasons. J Ayurveda Integr Med. 2014;5(4):251-259. DOI PubMed PMC Google Scholar Link
- Singh V, Amdekar S, Verma O. Ocimum Sanctum (tulsi): Bio-pharmacological Activities. Published online October 22, 2010. Accessed August 22, 2021. DOI Google Scholar Link
- Nadig P, Laxmi S. Study of anti-tussive activity of Ocimum sanctum Linn in guinea pigs. Indian J Physiol Pharmacol. 2005;49(2):243-245. PubMed Google Scholar Link
- Singh DP, Tripathi PK, Shalini T, Verma NK, Chandra V, Asha R. Phytochemical constituents and pharmacological activities of Ocimum sanctum (Tulsi): a review. Journal of Pharmaceutical Research and Clinical Practice. 2012;2(1):118-126. Google Scholar Link
- Mahajan N, Rawal S, Verma M, Poddar M, Alok S. A phytopharmacological overview on Ocimum species with special emphasis on Ocimum sanctum. Biomedicine & Preventive Nutrition. 2013;3(2):185-192. DOI Google Scholar Link
- Kumar A, Rahal A, Chakraborty S, Tiwari R, SK L, Dhama K. Ocimum sanctum (Tulsi): a miracle herb and boon to medical science - A Review. International Journal of Agronomy and Plant Production. 2013;4:1580-1589. Google Scholar Link
- Billah MM, Chowdhury AS, Nawrin K, Mostaq S, Rayhan MdA, Tushar RR. Serotonergic and noradrenergic response of ethanol extract; opioidergic response of ethyl acetate extract of Dicranopteris linearis L. leaf. Clinical Phytoscience. 2021;7(1):25. doi:10.1186/s40816-021-00262-8 DOI Google Scholar Link
- Rayhan MA, Vabna NJ, Ahmed F, Hossin A, Nawrin K, Billah MM. Jujube (Ziziphus jujube) honey treats stress induced anxiety behavior in mice. Pharmacotherapy and Pharmascience Discovery. 2021;1(1):1-9. DOI Google Scholar Link
- Miyagoshi M, Amagaya S, Ogihara Y. Antitussive effects of L-ephedrine, amygdalin, and makyokansekito (Chinese traditional medicine) using a cough model induced by sulfur dioxide gas in mice. Planta Med. 1986;(4):275-278. DOI PubMed Google Scholar Link
- Sinha S, Murugesan T, Pal M, Saha BP. Evaluation of anti-tussive activity of Bergenia ciliata Sternb. rhizome extract in mice. Phytomedicine. 2001;8(4):298-301. DOI PubMed Google Scholar Link
- Zhang J, Wang H, Chen C, et al. Addictive evaluation of cholic acid-verticinone ester, a potential cough therapeutic agent with agonist action of opioid receptor. Acta Pharmacol Sin. 2009;30(5):559-566. DOI PubMed PMC Google Scholar Link
- Liu W, Wang Y, He D, et al. Antitussive, expectorant, and bronchodilating effects of quinazoline alkaloids (±)-vasicine, deoxyvasicine, and (±)-vasicinone from aerial parts of Peganum harmala L. Phytomedicine. 2015;22(12):1088-1095. DOI PubMed Google Scholar Link
- Han N, Chang C, Wang Y, Huang T, Liu Z, Yin J. The in vivo expectorant and antitussive activity of extract and fractions from Reineckia carnea. J Ethnopharmacol. 2010;131(1):220-223. DOI PubMed Google Scholar Link
- Sulfur Dioxide Effects on Health - Air (U.S. National Park Service). Nps.gov. 2021. Accessed August 23, 2021. Link
- Wigenstam E, Elfsmark L, Bucht A, Jonasson S. Inhaled sulfur dioxide causes pulmonary and systemic inflammation leading to fibrotic respiratory disease in a rat model of chemical-induced lung injury. Toxicology. 2016;368-369:28-36. DOI PubMed Google Scholar Link
- Sulfur Dioxide. Medical Management Guidelines. Toxic Substance Portal. ATSDR. Accessed August 23, 2021. Link
- Jonasson S, Wigenstam E, Ågren L, Bucht A, Elfsmark L. Treatment of sulfur dioxide-induced lung injury in rats. European Respiratory Journal. 2017;50(suppl 61). DOI Google Scholar Link
- Meng Z, Qin G, Zhang B, et al. Oxidative damage of sulfur dioxide inhalation on lungs and hearts of mice. Environ Res. 2003;93(3):285-292. DOI PubMed Google Scholar Link
- Ren J, Ding X, Greer JJ. Mechanistic Studies of Capsaicin-Induced Apnea in Rodents. Am J Respir Cell Mol Biol. 2017;56(2):252-260. DOI PubMed Google Scholar Link
- Bolser DC, Davenport PW. Codeine and cough: an ineffective gold standard. Curr Opin Allergy Clin Immunol. 2007;7(1):32-36. DOI PMC Google Scholar Link
- Menezes PMN, Brito MC, de Sá PGS, Ribeiro LA de A, Rolim LA, Silva FS. Analytical and pharmacological validation of the quantification of phenol red in a mouse model: An optimized method to evaluate expectorant drugs. J Pharmacol Toxicol Methods. 2019;98:106586. DOI PubMed Google Scholar Link
- Engler H, Szelenyi I. Tracheal phenol red secretion, a new method for screening mucosecretolytic compounds. J Pharmacol Methods. 1984;11(3):151-157. DOI PubMed Google Scholar Link
- Rogers DF. Mucoactive agents for airway mucus hypersecretory diseases. Respir Care. 2007;52(9):1176-1193; discussion 1193-1197. Google Scholar Link
- Rubin BK. Mucolytics, expectorants, and mucokinetic medications. Respir Care. 2007;52(7):859-865. PubMed Google Scholar Link
- Zanasi A, Mazzolini M, Kantar A. A reappraisal of the mucoactive activity and clinical efficacy of bromhexine. Multidiscip Respir Med. 2017;12:7. DOI PubMed PMC Google Scholar Link
- Balsamo R, Lanata L, Egan CG. Mucoactive drugs. Eur Respir Rev. 2010;19(116):127-133. DOI PubMed Google Scholar Link
- Smith SM, Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database of Systematic Reviews. 2014(11). DOI PubMed PMC Google Scholar Link
- Yuta A, Baraniuk JN. Therapeutic approaches to mucus hypersecretion. Curr Allergy Asthma Rep. 2005;5(3):243-251. DOI PubMed Google Scholar Link
- Neame M, Aragon O, Fernandes RM, Sinha I. Salbutamol or aminophylline for acute severe asthma: how to choose which one, when and why? Arch Dis Child Educ Pract Ed. 2015;100(4):215-222. DOI PubMed Google Scholar Link
- Bennett WD. Effect of beta-adrenergic agonists on mucociliary clearance. J Allergy Clin Immunol. 2002;110(6 Suppl):S291-297. DOI PubMed Google Scholar Link
- Fazio F, Lafortuna C. Effect of inhaled salbutamol on mucociliary clearance in patients with chronic bronchitis. Chest. 1981;80(6 Suppl):827-830. DOI PubMed Google Scholar Link
- Ong HX, Traini D, Ballerin G, et al. Combined inhaled salbutamol and mannitol therapy for mucus hyper-secretion in pulmonary diseases. AAPS J. 2014;16(2):269-280. DOI PubMed PMC Google Scholar Link
- Jaggi RK, Madaan R, Singh B. Anticonvulsant potential of holy basil, Ocimum sanctum Linn., and its cultures. Indian J Exp Biol. 2003;41(11):1329-1333. PubMed Google Scholar Link
- Kelm MA, Nair MG, Strasburg GM, DeWitt DL. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7(1):7-13. DOI PubMed Google Scholar Link
- Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63(15):4375-4383. PubMed Google Scholar Link
Update History
| Revision Number | Date | Details Of Changes |
|---|---|---|
| 01 | 2021-11-11 | Original Article; published at its accepted version (Reference Number: PPD/MIN/2171A) |
 Pharmacotherapy & Pharmascience Discovery
Pharmacotherapy & Pharmascience Discovery
