- Received On: 2021-08-24|
- Accepted On: 2021-09-13|
- Published On: 2021-09-15
| Article Metadata | |||
|---|---|---|---|
| 1 | Submitted Manuscript | PPD/MIN/219/1 | |
| 2 | Cover Letter to Editor | PPD/CLE/219/1 | |
| 3 | Copyright Transfer Letter | PPD/CTL/219/1 | |
| 4 | Authors’ Consent Letter | PPD/ACL/219/1 | |
| 5 | Initial Editorial Screening Report | PPD/IESR/219/1 | |
| 6 | Review Agreement Letter (Reviewer 1) | PPD/RAL/2191/R1 | |
| 7 | Review Agreement Letter (Reviewer 2) | PPD/RAL/2191/R2 | |
| 8 | Manuscript Review Report (Round 1, Reviewer 1) | PPD/MRR/2191/R1.1 | |
| 9 | Manuscript Review Report (Round 1, Reviewer 2) | PPD/MRR/2191/R1.2 | |
| 10 | Revised Manuscript | PPD/MIN/2191R | |
| 11 | Review Response Letter (Round 1) | PPD/RRL/2191/R1 | |
| 12 | Manuscript Review Report (Round 2, Reviewer 1) | PPD/MRR/2191/R2.1 | |
| 13 | Manuscript Review Report (Round 2, Reviewer 2) | PPD/MRR/2191/R2.2 | |
| 14 | Final Editorial Screening Report | PPD/FESR/2191 | |
| 15 | Letter of Acceptance and Acknowledgement | PPD/LAA/2191 | |
| 16 | Accepted Manuscript | PPD/MIN/2191A | |
| 17 | Galley Proof Manuscript | PPD/MIN/GPM/2191 | |
| Request Access | |||
| Supplementary Data | ||
|---|---|---|
| The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request. | ||
| Datasets | Not Provided | Request Availability |
Abstract
Background: Antibacterial properties of black seed honey, mustard honey and lychee honey have got scientifically acknowledged through laboratory investigation and thus, found in many literatures. Though their mixture is believed to have enhanced potential, no scientific attempt was made on evaluation of such mixture. The aim of the present study was therefore to not only screen the antibacterial efficacy but also to assess their potential to revive the weak antibiotics against resistant bacteria.
Methods: All three honey were uniformly mixed to generate a 1:1:1 honey formulation (FH) and paired it with penicillin (FH-P) and amoxiclav (FH-A/C) as separate combinations. Two gram-positive and two gram-negative resistant bacteria were collected from clinical isolates. Bacterial susceptibility was evaluated by agar well diffusion method and percentage of bacterial growth inhibition was observed through microdilution technique. Furthermore, the minimum inhibitory and bactericidal concentration was determined.
Results: The formulated honey showed an upward efficacy with dilution which demonstrated significant potential of honey in bacterial growth inhibition. FH-A/C displayed rigorous synergism against K. pneumonia, S. aureus and S. pyogenes but failed against A. baumannii. On the contrary, FH-P exhibited facilitated action of penicillin only against S. pyogenes.
Conclusion: The study confirmed that the efficacy of formulated mixture of three selected honey could be utilized for further investigation which may generate clues for natural adjuvant therapy with optimum formulation.
Introduction
Antibiotic resistance, a global health care issue, has raised a deep concern among the researchers, practitioners and the patients. While new bacteria are evolving at a pace, we are stumbling to tackle the existing bacteria due to their developing resistant pattern. The threat gets immense aided by the shortage of new antibiotics in the pipeline which to serve the immediate requirement. Uncountable reports have already confirmed the failure of penicillin and amoxicillin-clavulanic acid (amoxiclav) to treat the common infectious bacteria [1-3]. However, reports have also indicated that the newly evolved or mutated bacteria can be susceptible to these basic drugs [4,5]. If such potentials are confirmed, it will save both time and cost as well as open up scopes for new research. Therefore, it has become a time-demand to investigate a suitable way to revive these antibiotics. Utilizing natural agents as adjuvant for antibiotics could be an easiest one.
Antibacterial properties of raw honey have been well recognized in modern medical science. However, the degree of potential varies mostly upon the sources – the flowers. Raw honey is acknowledged after the name of the flower from which bees collect the nectar. Black seed honey, mustard honey and lychee honey are the most popular of them due to their pleasant taste as well as medicinal values [6]. Literatures have confirmed strong antibacterial efficacy of these three honeys against a wide range of bacteria [7-8]. Moreover, black seed honey and litchi honey were also reported to have synergistic effect on combined application with penicillin and amoxicillin-clavulanic acid [9,10]. Whereas, recent approaches in antibacterial research have been made to evaluate natural adjuvant therapy to boost the action of weak antibiotics against resistant bacteria [11], no attempt has been initiated to formulate honey mixture so as to act as an optimized natural adjuvant for those antibiotics. The present study thus, aimed to evaluate a mixture of black seed honey, mustard honey and lychee honey in facilitating the action of penicillin and amoxiclav against resistant bacteria.
Methods
METHODS
Collection and preparation of the sample
Black seed (Nigella sativa) honey, mustard (Brassica Nigra) honey and lychee (Litchi chinensis) honey were collected from the honey cultivators of Dinajpur (25.63°N, 88.65°E), Tangail (24°13′N 90°3′E) and Sunamganj districts (25.0715°N, 91.3992°E) of Bangladesh respectively. Each type of honey was taken at 250ml volume. The samples were sieved with a 0.5 mm mesh to separate the coarse material, pollen, bees wax and any other bee product or impurities. The filtered honey was then kept in an air-sealed glass container to protect the honey from accumulation of moisture on the surface. For the experiments, 50ml of each honey type were homogenously mixed with a ratio of 1:1:1, termed as formulated honey (denoted as FH).
Characterization of the samples
Physicochemical properties of the collected honeys were assessed qualitatively according to the standard procedures in previous studies [12-14].
Collection of bacterial isolates
Two gram-negative and two gram-positive resistant clinical isolates were obtained from the Center for Medical Biotechnology, Institute of Public Health, Bangladesh. The collected strains were Klebsiella pneumonia (ATCC 13883), Acinetobacter baumannii (ATCC 17978), Staphylococcus aureus (ATCC 6538) and Streptococcus pyogenes (ATCC 19615) isolated from clinical samples. The bacteria were overnight incubated in Muller Hinton Agar (MHA) and Nutrient Broth (NB) and preserved in 15% glycerol.
Bacterial Susceptibility Test
The well diffusion assay was employed to investigate bacterial susceptibility towards the test agents [15,16]. Two freshly prepared MHA plates (90mm) were use in this regard where bacterial suspension (107 CFU per ml) were uniformly streaked over using sterile cotton swab. Each plate was divided into four quadrants where an 6mm well was cut in each quadrant using sterile cork borer. The first plate was subjected to wells contained 20μl of the test samples as follows – sterile water (control), phenoxymethylpenicillin [Sanofi Aventis (BD) Ltd.], ampicillin-clavulanic acid [Sanofi Aventis (BD) Ltd.] and honey mixture. The second plate followed wells for control, blank, penicillin-formulated honey combination and amoxiclav-formulated honey combination. The plates were then set to incubation for 24h at 37°C ± 1°C and afterwards, the zones over each well were measured using a vernier calipers scale. The test was repeated three times to consider the variability.
Microdilution Assay
The method was carried out with six dilutions of the formulated honey in a 96-well microplate [17]. The stock mixture was considered as of 100% strength and two-fold serial dilution were performed to obtain 50%, 25%, 12.5%, 6.25% and 3.125% strengths. These strengths were tested individually and in separate combination with penicillin and amoxiclav against the resistant isolates. Each well contained 10μl of bacterial suspension and 20μl of penicillin/amoxiclav/formulated honey in 300μl of NB, whereas, combination well contained 10μl of formulated honey and 10μl of penicillin/amoxiclav in place of individual test agent. The initial absorbance (T0) of the test suspension was determined at 630nm using a microplate reader (Biobase EL-10A, China) and kept for incubation at 37°C ± 1°C. After 24h, another absorbance (T24) was taken at the same wavelength. The difference of the two (T24 – T0) was considered the absorbance for bacterial growth (Abs) from which the percentage of bacterial inhibition was calculated comparing with the same of control using following formula:
Percentage inhibition = 1 − (Abs. test -Abs. control ) × 100
The method was performed in triplicate.
Minimum Inhibitory and Bactericidal Concentrations (MICs & MBCs)
From the microplate of microdilution assay, MIC was recorded by observing the wells with no visual turbidity at lowest concentration [10]. The observed well and its successive one (where applicable) were transferred to a fresh MHA plate and incubated for another 24h at 37°C ± 1°C. If the sample produced no visual colony was considered as MBC [18].
Statistical Analysis
Data were expressed as mean (n=3) ± standard deviation. To compare all groups with the control Dunnett t test was performed. The statistical analysis was carried out with one-way ANOVA test using SPSS v.24 where a p value less than 0.05 was considered significant.
Results
From agar well diffusion assay, it was observed that honey tended to show better efficacy in lower strengths following a linear dilution-dependent increase pattern (Figure 1a-1c). The same was observed in microdilution assay too (Figure 2a-2b, 3a-3b).
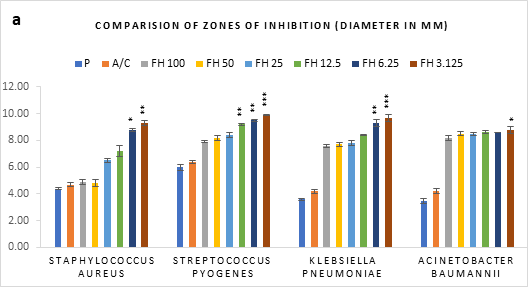
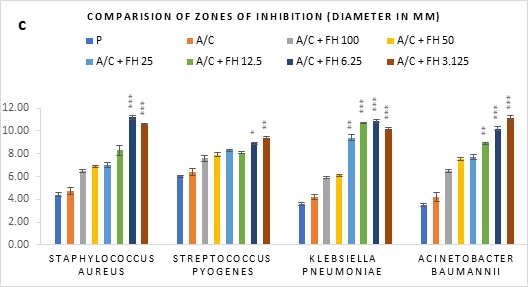
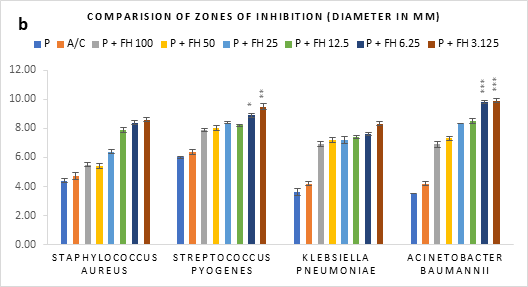
Figure 1 (a-c): Zones of inhibition of bacterial growth by (a) formulated honey and its combinations with standards (b) penicillin (c) amoxiclav, in agar well diffusion method. P = Penicillin; A/C = Amoxicillin + Clavulanic Acid; FH = Formulated mixture of black seed, mustard and litchi honey. Data represented as mean ± standard deviation, (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001; Dunnett t-test (two sided).
In agar well diffusion assay, the formulated honey in its individual use exhibited largest zone of inhibition against S. pyogenes (9.9mm at 3.125%) followed by K. pneumoniae (9.7mm at 3.125%). When applied together with penicillin, the combination worked best against A. baumannii (9.9mm, 3.125%) in comparison to its individual application (8.8mm, 3.125%). However, the conjugation found to reduce the inhibition of other three bacteria in comparison to that of formulated honey itself. On the contrary, amoxiclav-formulated honey coupling displayed synergistic antibacterial potential maximum on S. aureus (11.2mm, 6.25%), K. pneumoniae (10.9mm, 6.25%), A. baumannii (11.1mm, 3.125%) but except for S. pyogenes (9.4mm, 3.125%) where it was found to reduce the activity. Against all bacteria, penicillin and amoxiclav showed negligible inhibition.
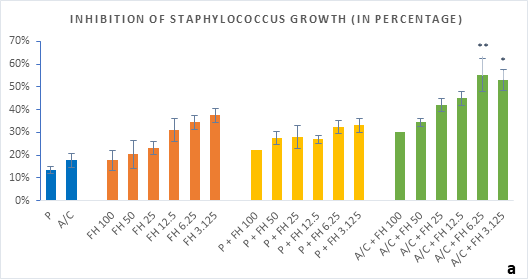
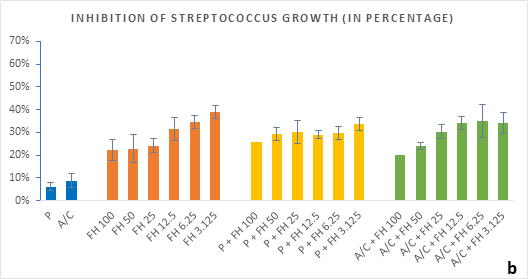
Figure 2 (a-b): Percentage of inhibition of bacterial growth (gram-positive) by formulated honey and its combinations with standards in microdilution method. (a) Staphylococcus aureus (b) Streptococcus pyogenes. P = Penicillin; A/C = Amoxicillin + Clavulanic Acid; FH = Formulated mixture of black seed, mustard and litchi honey. Data represented as percentage of inhibition as mean ± standard deviation, (n = 3); *p < 0.05, **p < 0.01; Dunnett t-test (two sided).
In microdilution assay, percentage of inhibition for individual bacterial growth was calculated. In case of S. aureus (Figure 2a), the formulated honey demonstrated a higher potential in inhibition with amoxiclav (55%, 6.25%) than individual use (37%, 3.125%) though, with penicillin contrarily decreased the potential (33%, 3.125%). But against S. pyogenes, despite the fact that FH produced higher inhibition (39%, 3.125%) comparing to that of S. aureus, could not exhibit such potential with amoxiclav (35%, 6.25%). K. pneumoniae growth was found to firmly inhibited by FH at 3.125% (42%) and FH-Amoxiclav at the same strength (51%). When paired with penicillin, both S. pyogenes and K. pneumoniae showed less susceptibility towards it than the FH itself. However, penicillin’s action was facilitated by FH only against A. baumannii at 3.125% (42%) which was higher than FH’s maximum efficacy (35%, 12.5%) as individual agent. Alongside, the FH-Amoxiclav combination also peaked at 12.5% (56%). Penicillin and amoxiclav could demonstrate mild efficacy against gram-positive bacteria only.
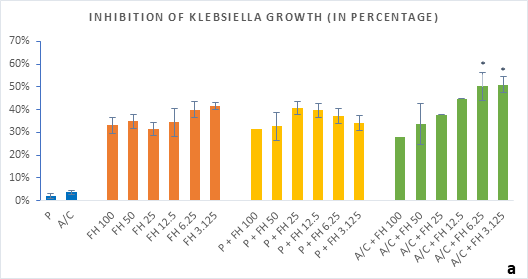
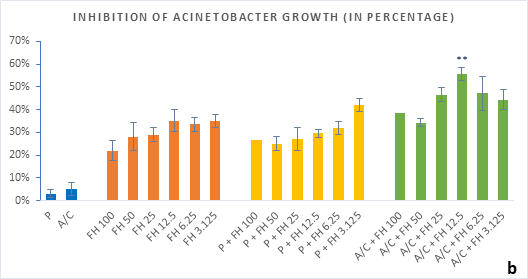
Figure 3 (a-b): Percentage of inhibition of bacterial growth (gram-negative) by formulated honey and its combinations with standards in microdilution method (a) Klebsiella pneumoniae (b) Acinetobacter baumannii. P = Penicillin; A/C = Amoxicillin + Clavulanic Acid; FH = Formulated mixture of black seed, mustard and litchi honey. Data represented as percentage of inhibition as mean ± standard deviation, (n = 3); *p < 0.05, **p < 0.01; Dunnett t-test (two sided).
Table 1 shows the MIC and MBC values against the different bacterial strains. With A/C+FH combinations the MIC was achieved at 3.125% for all except A. baumannii (6.25%). MBC for A. baumannii was recorded at 12.5% whereas MBCs for both K. pneumoniea and S. aureus were found at 6.25% FH. No MIC or MBC were obtained for S. pyogenes either with the standards.
Table 1: Determination of MIC and MBC against test bacteria.
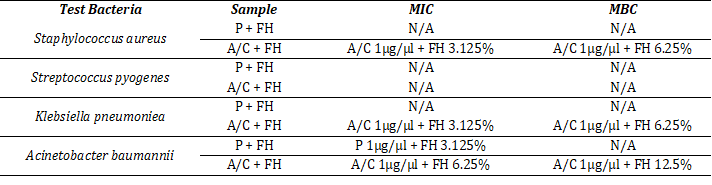
Data represent the minimum concentrations, at which the applied test samples showed inhibitory and bactericidal effect against the tested bacteria. N/A = No effect, P+FH = Combination of penicillin and mixed honey, A/C+FH = Combination of amoxicillin–clavulanic acid and mixed honey. The other test conditions (P, A/C, and FH alone) did not have an effect.
Discussion
Findings suggests that the formulated honey possesses a dilution-gradient efficacy relationship with lower strength demonstrating higher efficacy, which supported previous studies [19]. Moreover, it can be confirmed that the degree of antibacterial potential exhibited by this mixture of honey was noteworthy. Regarding the combinations, FH-Penicillin showed synergistic efficacy against A. baumannii but not against other bacteria. On the contrary, FH-Amoxiclav displayed similar action against all bacteria except S. pyogenes.
Honey is a complex natural antibacterial agent consisting of contributory factors like osmolarity [20,21], hydrogen peroxide [22], polyphenols [23], antioxidants [24] antibiotic peptides [25], and Maillard reaction products [24]. In diluted honey, glucose oxidase (GOX) enzyme generates hydrogen peroxide, which interacts with bacterial cell proliferative signals and eventually prevent bacterial growth [26-28]. Moreover, the antibacterial properties have been attributed to Def-1, an antibacterial peptide, possessing broad spectrum antibiotic effect [26]. The antibacterial activity of honey is also credited to the presence of other constituents like methylglyoxal (MGO) and its precursor dihydroxyacetone (DHA) [29].
Thymoquinone and melanin are the main active components of black seed honey that contribute to its antimicrobial property [30]. Honey derived from mustard also possesses certain pharmacological properties that are attributed to its nutritional elements- polyphenol, vitamin C, flavonoids and certain heavy metals [31]. Similar compounds apart from antioxidants have been also reported for litchi honey which may have contributed to its antibacterial properties [32].
Beta-lactam antibiotics exerts their action by blocking transpeptidase enzyme, a precursor for cross linkage of peptidoglycan in both gram-positive and gram-negative bacteria [33]. Consequently, cell becomes ruptured due to notwithstanding the osmotic pressure [34]. Resistance to penicillin (once most effective β-lactam antibiotic) occurs due to the target modification of peptidoglycan-binding-proteins in gram-positive bacteria [35], while gram-negative bacteria possessing a double membrane resists the entry of penicillin across it [36]. Moreover, it hydrolyzes the β-lactam ring [36]. A third-generation penicillin, amoxicillin, used to show higher stability to β-lactamase and perform with better efficacy [34]. However, it also is not out of the scope of cleavage of beta-lactam ring by the bacteria. Combination of amoxicillin and clavulanic acid (beta-lactamase inhibitor). As like the inherent characteristics of clavulanic acid of not having any direct bactericidal properties [37], some compounds of honey could have contributed in similar mechanism to obtain the reverse effect of penicillin and amoxiclav.
Conclusion
The formulated mixture of these three honeys remarkably inhibited both the gram-positive and gram-negative bacteria. its combination with penicillin and amoxiclav also proved to have synergistic potential, however, at this stage of the study the underlying mechanism is unknown. Further investigation shall be undertaken to identify the responsible bioactive compounds.
Abbreviations
PEN: Penicillin; A/C = Amoxicillin + Clavulanic Acid; FH = Formulated mixture of black seed, mustard and litchi honey; MHA: Muller Hinton Agar; NB: Nutrient Broth; ATCC: American Type Culture Collection.
Ethics Approval and Consent to Participate
The work was exempt for ethical approval. All experiments were performed in accordance with the Institutional Standard Microbial Testing Procedures (Ref. no. IPSDRLAB/ ISMTP/01/19) of Institute for Pharmaceutical Skill Development and Research, BD.
Acknowledgments
The present study was supported and carried out in the Research lab of Institute for Pharmaceutical Skill Development and Research, Bangladesh. Authors are also grateful to the Center for Medical Biotechnology under MIS, DGHS, for providing the bacterial strains used in this study.
Authors’ Contributions
This work was carried out in collaboration between both authors, contributed equally.
Consent for Publication
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study was carried out with individual funding of all authors.
Availability of Data and Materials
Available from corresponding author on valid request.
Competing Interests
Authors agreed on the article before submission and had no conflict of interests.
References
- Pichichero ME, Casey JR, Mayes T, et al. Penicillin failure in streptococcal tonsillopharyngitis: causes and remedies. Pediatr Infect Dis J. 2000;19(9):917-923. DOI PubMed Google Scholar Link
- Sela S, Barzilai A. Why do we fail with penicillin in the treatment of group A streptococcus infections? Ann Med. 1999;31(5):303-307. DOI PubMed Google Scholar Link
- Block SL. Strategies for dealing with amoxicillin failure in acute otitis media. Arch Fam Med. 1999;8(1):68-78. DOI PubMed Google Scholar Link
- d'Alessio V. Can we reverse antibiotic resistance? Research and Innovation. 2019. Accessed September 12, 2021. Link
- Qadri SMH, Ueno Y. Susceptibility of Pathogenic Bacteria to Amoxicillin/Clavulanic Acid (Augmentin) at a Referral Hospital. Annals of Saudi Medicine. 1989;9(5):448-451. DOI Google Scholar Link
- Linkon MR, Prodhan UK, Elahi T, Talukdar J, Alim MA. Comparative Analysis of the Physico-chemical and Antioxidant Properties of Honey Available in Tangail, Bangladesh. Universal Journal of Food and Nutrition Science. 2015;3(1):19-22. DOI Google Scholar Link
- Khalil MI, Motallib MB, Anisuzzaman ASM, Sathi ZS, Hye MA, Shahjahan M. Antibacterial Activities of Different Brands of Unifloral Honey Available at the Northern Region of Bangladesh. Journal of Medical Sciences. 2001;1(6):389-392. DOI Google Scholar Link
- Almasaudi SB, Al-Nahari AAM, Abd El-Ghany ESM, et al. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi Journal of Biological Sciences. 2017;24(6):1255-1261. doi:10.1016/j.sjbs.2016.08.007 DOI PubMed Google Scholar Link
- Hossain R, Rahman MS, Rayhan MA, Nawrin K, Billah MM, Habib MR. Antibacterial efficacy of black seed honey in combination with penicillin and amoxiclav against gram-positive bacteria. International Journal of Scientific Reports. 2020;6(2):61-66. DOI Google Scholar Link
- Vabna NJ, Sultana N, Khan M, Ghosh B, Nawrin K, Billah MM. Lychee Honey Accelerates Antibacterial Efficacy of Penicillin and Amoxiclav Against Gram-Positive Bacteria. AJBSR. 2019;6(3):226. DOI Google Scholar Link
- Liu A. Making drug-resistant bacteria susceptible to antibiotics. Fierce Biotech. 2021. Accessed September 12, 2021. Link
- Billah MM, Rayhan MA, Yousuf SA, Nawrin K, Hossin A, Khengari EM. Integrated OF-HC-EPM approach to assess sedative-anxiolytic potential of honey derived from mustard (Brassica nigra) flower. International Journal of Scientific Reports. 2019;5(8):201-206. DOI Google Scholar Link
- Rayhan MA, Yousuf SA, Rayhan J, Khengari EM, Nawrin K, Billah MM. Black seed honey - A powerful ingredient of prophetic medicine; its neuropharmacological potential. Journal of Apitherapy. 2019;5(2):18-26. DOI Google Scholar Link
- Billah MM, Rayhan MdA, Yousuf SA, Nawrin K, Jahan N, Khengari EM. A Novelty-Induced Integrated Environmental Challenge to Assess Sedative-Anxiolytic Potential of Lychee (Litchi Chinensis) Honey. Am J Biomed Sci. Published online July 2019:120-129. DOI Google Scholar Link
- Stagos D, Soulitsiotis N, Tsadila C, Papaeconomou S, Arvanitis C, Ntontos A, et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int J Mol Med. 2018;42(2):726–34. DOI PubMed PMC Google Scholar Link
- Eid AM, Istateyeh I, Salhi N, Istateyeh T. Antibacterial activity of fusidic acid and sodium fusidate nanoparticles incorporated in pine oil nanoemulgel. Int J Nanomedicine. 2019;14:9411–21. DOI PubMed PMC Google Scholar Link
- Fani M, Kohanteb J. In Vitro Antimicrobial Activity of Thymus vulgaris Essential Oil Against Major Oral Pathogens. J Evidence-Based Complement Altern Med. 2017;22(4):660–6. DOI PubMed PMC Google Scholar Link
- Khan M, Islam Z, Chowdhury AS, Yousuf SA, Amin MR, Rayhan MA. Wild Honey Facilitates Antibacterial Efficacy of Penicillin and Amoxicillin-Clavulanic Acid. Journal of Apitherapy. 2020;7(2):22–30. DOI Google Scholar Link
- Billah MM, Nawrin K, Rayhan MA, Ahmed F, Hossin A, Islam MN. Honeys from diverse geographical sources supplement the action of penicillin and amoxiclav – a comparative antibacterial study. Pharmacotherapy & Pharmascience Discovery. 2021; 1(1): 19-28. DOI Google Scholar Link
- Wahdan HAL. Causes of the antimicrobial activity of honey. Infection 1998; 26: 26-31. DOI Google Scholar Link
- Bose B. Honey or sugar in treatment of infected wounds? Lancet 1982; 1(8278): 963. DOI PubMed Google Scholar Link
- Brudzynski K, Abubaker K, Miotto D. Unraveling a mechanism of honey antibacterial action: Polyphenol/H2O2-induced oxidative effect on bacterial cell growth and on DNA degradation. Food Chem 2012; 133: 329-336. DOI PubMed Google Scholar Link
- Aljadi AM, Yusoff KM. Isolation and identification of phenolic acids in Malaysian honey with antibacterial properties. Turk J Med Sci 2003; 3: 229-236. Google Scholar Link
- Brudzynski K, Miotto D. Honey melanoidins. Analysis of a composition of the high molecular weight melanoidin fractions exhibiting radical scavenging capacity. Food Chem 2011; 127: 1023-1030. DOI PubMed Google Scholar Link
- Kwakman PHS, de Boer L, Ruyter-Spira CP, Creemers-Molenaar T, Helsper JPFG, Vandenbroucke-Grauls CMJE, et al. Medicalgrade honey enriched with antimicrobial peptide has enhanced activity against antibioticresistant pathogens. Eur J Clin Microbiol Infect Dis 2011; 30: 251-257 DOI PubMed PMC Google Scholar Link
- Bucekova M, Jardekova L, Juricova V, Bugarova V, Di Marco G, Gismondi A, et al. Antibacterial activity of different blossom honeys: New findings. Molecules. 2019;24(8). DOI PubMed PMC Google Scholar Link
- Irish J, Blair S, Carter DA. The antibacterial activity of honey derived from Australian flora. PLoS One. 2011;6(3):18229. DOI PubMed PMC Google Scholar Link
- Tan HT, Rahman RA, Gan SH, Halim AS, Hassan SA, Sulaiman SA, et al. The antibacterial properties of Malaysian tualang honey against wound and enteric microorganisms in comparison to manuka honey. BMC Complement Altern Med. 2009;9:34. DOI PubMed PMC Google Scholar Link
- Grainger MNC, Manley-Harris M, Lane JR, Field RJ. Kinetics of conversion of dihydroxyacetone to methylglyoxal in New Zealand mānuka honey: Part I – Honey systems. Food Chemistry. 2016;202:484-491. DOI PubMed Google Scholar Link
- Forouzanfar F, Fazly Bazzaz BS, Hosseinzadeh H. Black cumin (Nigella sativa) and its constituent (thymoquinone): A review on antimicrobial effects. Iran J Basic Med Sci. 2014; 17(12):929–38. DOI PubMed PMC Google Scholar Link
- Cokcetin NN, Pappalardo M, Campbell LT, Brooks P, Carter DA, Blair SE, et al. The antibacterial activity of Australian Leptospermum honey correlates with methylglyoxal levels. PLoS One. 2016;11(12). DOI PubMed PMC Google Scholar Link
- Islam MS, Jothi JS, Islam M, Zubair MA. Antioxidant and Physico-Chemical Properties of Litchi Honey Procured from Gazipur and Tangail District, Bangladesh. JEZS. 2014; 2 (5): 207-211 Google Scholar Link
- Bush K, Bradford PA. β-lactams and β-lactamase inhibitors: An overview. Cold Spring Harb Perspect Med. 2016;6(8). DOI PubMed PMC Google Scholar Link
- Lobanovska M, Pilla G. Penicillin’s discovery and antibiotic resistance: Lessons for the future? Yale J Biol Med. 2017;90(1):135–45. PubMed PMC Google Scholar Link
- Fisher JF, Mobashery S. β-Lactam resistance mechanisms: Gram-positive bacteria and mycobacterium tuberculosis. Cold Spring Harb Perspect Med. 2016;6(5). DOI PubMed PMC Google Scholar Link
- Livermore DM. Current epidemiology and growing resistance of Gram-negative pathogens. Korean J Intern Med. 2012; 27(2):128–42. DOI PubMed PMC Google Scholar Link
- Akhavan BJ, Khanna NR, Vijhani P. Amoxicillin. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 27, 2020. PubMed PMC Google Scholar Link
Update History
| Revision Number | Date | Details Of Changes |
|---|---|---|
| 1 | 2021-09-15 | Original Article; published at its accepted version (Reference Number: PPD/MIN/2191A) |
 Pharmacotherapy & Pharmascience Discovery
Pharmacotherapy & Pharmascience Discovery
