- Received On: 2022-09-22|
- Accepted On: 2022-11-14|
- Published On: 2022-11-25
| Article Metadata | |||
|---|---|---|---|
| 1 | Submitted Manuscript | PPD/MIN/22212/1 | |
| 2 | Cover Letter to Editor | PPD/CLE/22212/1 | |
| 3 | Copyright Transfer Letter | PPD/CTL/22212/1 | |
| 4 | Authors’ Consent Letter | PPD/ACL/22212/1 | |
| 5 | Initial Editorial Screening Report | PPD/IESR/22212/1 | |
| 6 | Review Agreement Letter (Reviewer 1) | PPD/RAL/22212/R1 | |
| 7 | Review Agreement Letter (Reviewer 2) | PPD/RAL/22212/R2 | |
| 8 | Manuscript Review Report (Round 1, Reviewer 1) | PPD/MRR/22212/R1.1 | |
| 9 | Manuscript Review Report (Round 1, Reviewer 2) | PPD/MRR/22212/R1.2 | |
| 10 | Revised Manuscript | PPD/MIN/22212R | |
| 11 | Review Response Letter (Round 1) | PPD/RRL/22212/R1 | |
| 12 | Manuscript Review Report (Round 2, Reviewer 1) | PPD/MRR/22212/R2.1 | |
| 13 | Manuscript Review Report (Round 2, Reviewer 2) | PPD/MRR/22212/R2.2 | |
| 14 | Final Editorial Screening Report | PPD/FESR/22212 | |
| 15 | Letter of Acceptance and Acknowledgement | PPD/LAA/22212 | |
| 16 | Accepted Manuscript | PPD/MIN/22212A | |
| Request Access | |||
| Supplementary Data | ||
|---|---|---|
| The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request. | ||
| Datasets | Not Provided | Request Availability |
Abstract
Background: Alzheimer's disease is a prevalent neurological condition that leads to dementia. The illness advances slowly through the destruction of neurons. Though loss of memory characterizes the disease, interestingly, recent reports also suggest motor coordination can influence or be affected by the disease. Ketamine is a common anesthetic medicine but its effect on nervous system is debatable. Most of the studies over ketamine’s effect on cognitive function were conducted with administration of high dose, whereas its potential at low dose remained undiscovered. Thus, the present study was aimed to assess the effect of low dose of ketamine as sole administration and in a combination with antioxidant on cognition and motor coordination in a mouse model of Alzheimer's disease.
Methods: Mice were given scopolamine for seven days to elicit Alzheimer-like symptoms such as loss of memory and motor coordination. Afterwards, they either received intraperitoneal dose of 5, 10, 20 mg/kg of ketamine only or along with an antioxidant (a complex of Betacarotene, vitamin-C, and vitamin-E) for cognitive and motor function test. The cognitive behavior was assessed using the Y maze test, modified hole cross test, and Morris water maze test, and motor coordination was evaluated using the rotatod and pole test.
Results: The study revealed that ketamine at 20 mg/kg body weight interferes with cognition and impairs motor coordination in Alzheimer's mouse model. Ketamine 10 mg/kg in combination with the antioxidant resulted in significant cognitive improvement however, exhibited marginal improvement in motor coordination. Though previously reported by other studies, antioxidant as a positive control could not produce significant effect on motor coordination as observed in this study.
Conclusion: More research at the cellular and molecular level is required to understand the mechanism behind conservation of cognitive function. Moreover, the synergistic potential of the combination (antioxidants and ketamine) in ameliorating cognitive effects is to be explored.
Significance Statement
The study obtained several new highlights contributable to its field; firstly, findings of the present study supported previous hypotheses of motor failure as a characteristic feature of Alzheimer’s disease; secondly, high dose of ketamine severely impacts both memory and motor function associated with AD, whereas, a moderate dose proves to be beneficial alone and in combination with antioxidant; thirdly, antioxidant ameliorates memory effectively but motor functions marginally. Hence, the study could could lead to ketamine-based combination therapy in Alzheimer’s patients for improving cognitive function.
Introduction
Alzheimer’s disease (AD) is a common neurological disease which is characterized by memory loss and motor coordination loss. The disease progresses slowly by damaging of brain cells. There are around 50 Million Alzheimer’s patients in the world which estimated to be tripled by 2050 [1]. Patients with AD mostly suffer by losing memory progressively, problem in doing daily life activities, sometimes problem with language or making sentences as well as loss of perception and motor function [2]. In terms of economic burden, total cost for care giver management, physician, medicine to other direct and indirect related cost is estimated around 1 trillion dollar worldwide [3]. In general, two proteins - amyloid-beta and tau buildup are linked to the progressive cognitive deterioration in AD [4]. Beta-secretase and gamma-secretase sequentially cleave the amyloid precursor protein (APP), resulting in the formation of amyloid-beta peptide. Thus, the aggregation of amyloid-beta peptide produces hazardous oligomers for the neurons [5]. Tau, however, forms when the microtubule-associated protein tau’s regulatory gene is alternatively spliced to create soluble protein isoforms [6]. A number of functional interactions between Aβ and tau protein have been linked to cognitive impairment and neuronal circuit destruction in AD [7,8]. Aging, infection, injury of brain, some genetic factors such as mutation of PSEN1, APP and PSEN2 genes, obesity, accumulation of heavy metals in brain and vascular disease are the most common risk factors for Alzheimer’s disease [9–12]. Moreover, interestingly, some studies reported that people with slower movement and poor balance are more likely to diagnose with AD in their following 6 year of age [13], while others confirmed motor failure at late stages of AD [14]. Therefore, the study hypothesized the possibilities of concurrence of both motor and memory impairment in pathologic condition of AD. Till date, there is no cure for this disease, however, some treatment options are there to slow down the disease [15].
Investigating the potential of some natural compounds with neuroprotective properties is one of the most recent approach for treating AD [16]. Currently, major two groups of drugs are approved to treat the disease, cholinesterase inhibitors which decrease the metabolism of acetyl choline resulting increase in acetyl choline and antagonists of NMDA (N-methyl D-aspertate) as NMDA over excitation causes neuronal death and various malfunction [16]. Additionally, antioxidant drugs are also prescribed for treating Alzheimer’s patient as oxidative stress causes formation of many of the toxic compounds which might create the risk of Alzheimer’s disease [17].
Ketamine has been a therapeutic choice to induce anesthesia. Recent studies indicate its potential as an antidepressant medicine [18]. Ketamine exerts its antidepressant effect via an increase in synaptic glutamate concentration [19]. Nonetheless, ketamine causes cognitive dysfunction and neuronal death at 30mg/kg or higher concentration in mice by reducing brain-derived neurotrophic factor (BDNF) drastically [20,21]. Despite such negative effect on cognitive function, interestingly ketamine is also an antagonist of NMDA receptor which leads to a hypothesis over utilizing it as an anti-Alzheimer’s disease medicine [22]. Furthermore, at low doses, ketamine has antioxidant activity, resulting in neuroprotective effects [23]. Thus, this study attempts to determine if motor failure also features Alzheimer’s disease alongside the characteristics of memory loss and if lower dose of ketamine, such as 5, 10, and 20 mg/kg, alone or in combination with antioxidant can enhance these performances in a mouse model of Alzheimer's disease.
Methods
Drugs and Chemicals
Commercially viable IV dosage of ketamine (50mg/ml) were procured from Popular Pharmaceuticals Ltd. (Bangladesh). In addition, antioxidant medication was bought from Square Pharmaceuticals Ltd. (Bangladesh) in tablet form, which comprises betacarotene, vitamin-C, and vitamin-E doses at 6, 200, and 50mg per tablet, respectively. From Opsonin Pharmaceuticals Ltd., 0.9% NaCl solution was purchased (Bangladesh).
Experimental Animal
For the studies, Swiss albino mice aged 45 days and weighing 25 to 30 grams were used. The animals were subjected to a 12h light/dark cycle, adequate air ventilation, and ambient temperature. The animals were housed in the animal home at the Institute for Pharmaceutical Skill Development and Research, Bangladesh, where they were given adequate water and food access.
Experimental Design
For the duration of the study, we separated mice into nine groups, each containing five. Except blank group, all mice were given scopolamine 1mg/kg orally, treated for 7 days to induce Alzheimer’s like behavior such as memory and motor impairment according Yadang et al. study in 2020 [24]. These groups were further subjected to the following treatments:
Group 1: Blank or completely healthy mice (saline water, i.p.), Group 2: Control or Alzheimer's mice (saline water, i.p.), Group 3: Antioxidant complex (betacarotene 1.2 mg/kg + vitamin-C 41 mg/kg + vitamin-E 10.3 mg/kg, p.o.), Group 4: Ketamine (5 mg/kg, i.p.), Group 5: Ketamine (10 mg/kg, i.p.), Group 6: Ketamine (20 mg/kg, i.p.), Group 7: Antioxidant complex (p.o.) + Ketamine (5 mg/kg, i.p.), Group 8: Antioxidant complex (p.o.) + Ketamine (10 mg/kg i.p.), Group 9: Antioxidant complex (p.o.) + Ketamine (20 mg/kg i.p.). I.p doses were given 30 minutes before the experiment and oral doses were given 1 hour before the experiment for all the studies conducted.
To evaluate cognitive function three tests were done, Y maze recognition memory test for short term memory assessment, modified hole cross test for spatial memory based on reward assessment and Morris water maze for spatial and working memory assessment. Moreover, to access Motor coordination rotatod and pole tests were conducted.
Y Maze Test
Y-maze was constructed of wood, wrapped in white sheets with various black patterns painted on top to provide visual cues, and had three arms with a 120° angle between each pair of arms. Each arm measured 8 x 30 x 15 centimeters (width x length x height). The arms were designated (i) start arm, where the mouse began exploring (always open), (ii) novel arm, which was restricted during the first trial but allowed access during the second trial, and (iii) the familiar arm was open for the entire period. In order to limit olfactory cues, the floor of the maze was coated with sawdust. To measure spatial recognition memory, the Y-maze test included two trials divided by an inter-trial interval (ITI). The first trial [training] lasted 10 minutes and enabled the mouse to explore only two arms of the maze (start arm and other arm), while the third arm (new arm) was blocked. After 1h of ITI, the second trial (retention) was done, in which the mouse was transferred back into the maze in the same beginning arm, with exposure to all three arms for 5 minutes. After the second trial, the relation between novelty and familiarity was calculated by comparing the time spend in all of the arms [25].
Modified Hole Cross Test
Mice were placed in a hole cross apparatus and permitted to freely traverse a 3 cm hole in a 7 cm-high barrier that splits the 30x20x14 cm box into two equal compartments. After crossing the hole from beginning compartment, a 500ml beaker was placed parallel to the ground in a way that only a tiny space next to the apparatus wall was available for the mice to enter inside the beaker and take the food kept inside the beaker. The test was done in two consecutive days and mice were fasted for 24 hours on both days of the study. In the first trial (training day), mice were put into the empty chamber and allowed 10 minutes to investigate the apparatus, cross the hole, and find their way to go inside the beaker by a narrow pathway to reach the food within the beaker. If a mouse did not reach the food after 10 minutes, it was excluded from the study. On the second trial (test day), mice were put identically to the first trial, and the time required to reach the food was measured. The shorter amount of time required to reach the food indicated enhanced memory or cognitive function.
Morris Water Maze Test
The water maze was formed on a pool with a diameter of approximately 6 feet and a depth of approximately 3 feet. The pool was filled with tap water and kept at 26 degrees Celsius. The escape platform was situated in the pool's middle which was submerged just beneath the surface of the water and was not visible to mice since milk has been added to make the water cloudy. The study involved two trials. The water maze included four possible beginning positions: north, south, east, or west. Animals were positioned in one of these places. Initially, the animals swum around the pool's perimeter in search of an exit. Eventually, the animal learnt to seek out and ascend to the platform. Once the mice reached the platform, they were expected to remain seated for 15 seconds. If it leaped off, it was gently led back to the platform. This taught the animal that in order to be retrieved from the pool, it must remain on the platform. For three trials, the identical technique was followed from four distinct directions, with each trial beginning in a different direction. Initially, mice were given 1 minute; if they failed, they were given an additional 1 minute; if they did not locate the platform within 3 minutes, they were excluded from the study. After the animals had completed all three tests, it was dried off with a towel. On the test day, each animal had 12 trials, three for each beginning direction. The platform discovery or escape latency time was assessed and recorded [26].
Rotarod Test
The rods were comprised of a strong plastic substance coated with a grey rubber foam. The diameter of the rotating rods was five centimeters. In 300 seconds, the equipment was permitted to accelerate from 4 rpm to 40 rpm. The rotarod's initial speed was 4 rpm, and its acceleration rate was 20 rpm/min. The mouse was held by its tail and put on the revolving rod with its back to the direction of rotation, requiring it to move forward to maintain its balance. The study consisted of three trials separated by 15-minute pauses (ITI). There was no training phase preceding the examination. The latency to fall, was measured manually [27].
Pole Test
The apparatus was 50-centimeter-tall, 0.5-centimeter-diameter, gauze-wrapped wooden pole topped with a wooden ball. The mice were trained initially three times with the pole to ensure that all of them would tilt their heads down when placed on the ball. During the test, the duration it took for the mice to travel from the top to the bottom of the apparatus was measured. Each mouse had three trials with 5-minute intervals between each. For statistical analysis, the mean of the three trials was calculated [28].
Statistical Analysis
All tests were statistically analyzed using a two-way ANOVA and Dunnett's multiple comparison test in GraphPad Prism 8. All test findings were compared to a control group to determine their statistical significance. P values of 0.05, 0.01, and 0.001 were deemed statistically significant and denoted with the symbols *, **, and ***, respectively.
Results
Y Maze Test
The Y maze test was carried out to evaluate acute memory of the test animal (Figure 1A). In a 300 second duration of test, Blank group (non-Alzheimer’s mice) exhibited higher duration of time (~185s) spent in novel arm whereas, control (untreated Alzheimer’s mice) refrained itself from such exploration. Antioxidant exposure sufficiently increased the duration (137s) which was mimicked by Ketamine 10 mg/kg and eventually, synergized in their combination (157s). The synergy was though evident in other ketamine doses, could not produce a significant difference from its individual use. Moreover, despite ketamine’s initial dose dependent increase in total time spent in novel arm by 5 and 10 mg/kg, a higher dose of 20 mg/kg drastically reduced the activity (22s).
Modified Hole Cross Test
The modified hole cross test was conducted to examine the spatial memory of mice. Data shows that mice treated with ketamine 20mg/kg alone had the most difficulty in locating food (132 seconds), followed by mice treated with ketamine 20mg/kg and antioxidant (94 secs). In contrast, blank or non-Alzheimer’s mice were able to locate the food in 14.6 seconds, followed by the antioxidant alone treated group in 42 seconds, the ketamine 10mg/kg alone and the ketamine 10mg/kg plus antioxidant treated group in 57 & 28 seconds respectively (Figure 1B).
Morris Maze Test
The Morris maze method was used to investigate the spatial and working memory of mice. The findings of the test (Figure 1C) revealed that mice treated with ketamine 20mg/kg alone required the longest time to locate the platform (131s). On the contrary, antioxidant itself was highly effective (25s) in reducing the parameter. In addition, the antioxidant in its combination with such high dose of ketamine (20 mg/kg) also lowered the response synergistically (68.4s) however, was not significant enough to alter the loss of memory compared to control (54 seconds). Nevertheless, blank group of mice were able to locate the platform quickly (9.2 seconds), followed by the combination of ketamine 10mg/kg and antioxidant-treated group (20 seconds) which was surprisingly more effective than the positive control itself.
A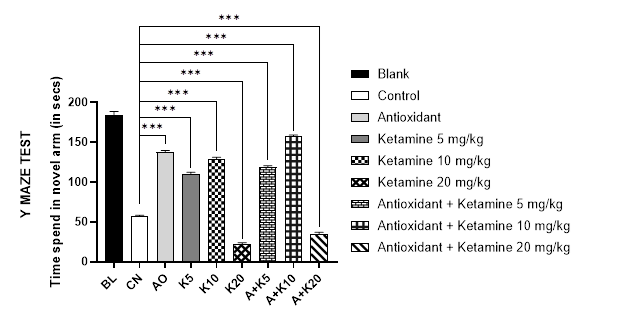
B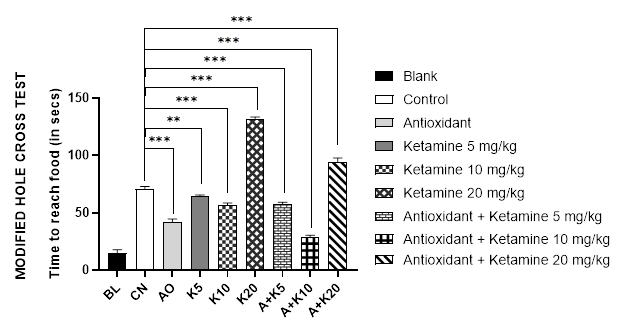
C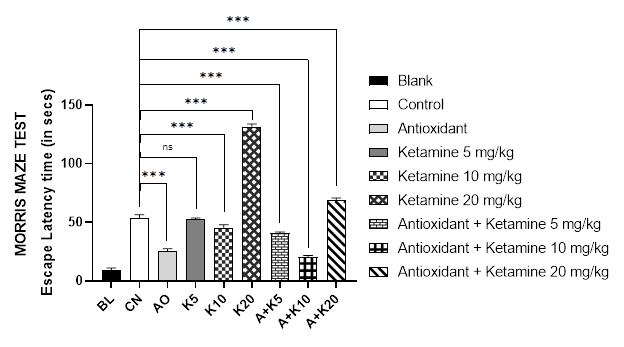
Figure 1 (A-C): Comparative cognition with ketamine-based therapies in Alzheimer’s mice (Assessed in 1A: Y-maze test, 1B: Modified hole cross test and 1C: Morris maze test).
BL = blank, CN = control, AO = antioxidant complex (betacarotene 1.2 mg/kg + vitamin-C 41 mg/kg + vitamin-E 10.3 mg/kg), K5/ K10/ K20 = ketamine at 5 mgkg-1/ 10 mgkg-1/ 20 mgkg-1 concentration, A + K5/ K10/ K20 = antioxidant complex + ketamine at 5 mgkg-1/ 10 mgkg-1/ 20 mgkg-1 concentration. Data illustrated as mean ± SEM, (n=10); ns = non-significant, * = p <0.05, ** = p <0.01, *** = p <0.001; Dunnett’s multiple comparison test was performed, where 0.9% saline treated Alzheimer’s mice group served as control and all other groups were compared against the control. All groups of mice except Blank were pre-treated with scopolamine (1mg/kg) for 7 days.
Rotarod Test
The rotarod test was performed to observe the mice's motor coordination. Figure 2A depicts the results of the rotarod test for different treatment groups. Data demonstrated that the ketamine 20mg/kg alone and ketamine 20mg/kg with antioxidant-treated groups of mice fell from the apparatus quickly (about 5.5 seconds). Nonetheless, blank mice were able to stay on the rotarod for longer duration (31 seconds), followed by the antioxidant alone and ketamine 10mg/kg combined with antioxidant treated group (around 15 seconds).
Pole Test
Like Rotarod test, the pole test was used to analyze the motor coordination of mice. The results of the pole test for various treatment groups are displayed in Figure 2B, where it was evident that mice of combined ketamine 20 mg/kg and antioxidant therapy group had the longest time to descend from the pole (31.2 seconds). In addition, ketamine 20mg/kg alone-treated mice took the second-longest time, 24.5 seconds. However, the blank group mice descended from the pole in 10 seconds, followed by antioxidant-only treated and antioxidant along with ketamine 10mg/kg treated animals in approximately 16 seconds which failed to demonstrates significant differences between the two groups.
A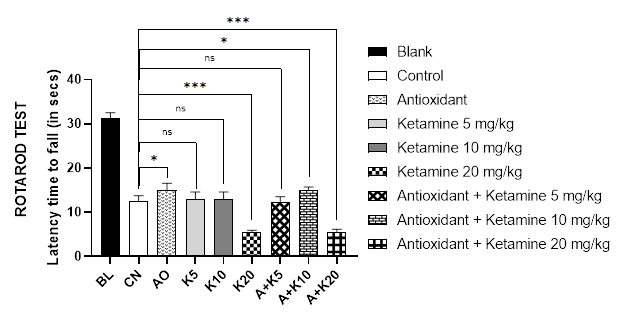
B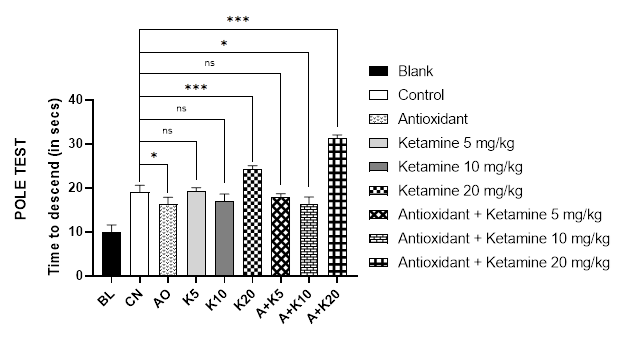
Figure 2 (A-B): Comparative motor coordination with ketamine-based therapies in Alzheimer’s mice (Assessed in 2A: Rotarod test and 2B: Pole test).
BL = blank, CN = control, AO = antioxidant complex (betacarotene 1.2 mg/kg + vitamin-C 41 mg/kg + vitamin-E 10.3 mg/kg), K5/ K10/ K20 = ketamine at 5 mgkg-1/ 10 mgkg-1/ 20 mgkg-1 concentration, A + K5/ K10/ K20 = antioxidant complex + ketamine at 5 mgkg-1/ 10 mgkg-1/ 20 mgkg-1 concentration. Data illustrated as mean ± SEM, (n=10); ns = non-significant, * = p <0.05, ** = p <0.01, *** = p <0.001; Dunnett’s multiple comparison test was performed, where 0.9% saline treated Alzheimer’s mice group served as control and all other groups were compared against the control. All groups of mice except Blank were pre-treated with scopolamine (1mg/kg) for 7 days.
Discussion
Investigating the molecular mechanisms behind amyloid- and tau pathology has led to major advances in the knowledge of Alzheimer's disease over the past few decades. Hyperphosphorylation of certain amino acids in tau proteins leads it to detach from microtubules, disrupting the transport mechanism, eventually leading to starvation of neurons and cell death [29]. Moreover, formation of amyloid plaques, neurofibrillary tangles, synapse loss leading neuronal cell death, is caused by excessive levels of Aβ peptide in the brain [30]. Death of neurons in hippocampus causes memory loss and subsequent damage to the integrity of the gray matter of motor-related brain areas can lead to motor impairment [31].
NMDA receptor is another attributable component in the development of Alzheimer’s disease. Glutamate, a NMDA receptors ligand is an excitatory neurotransmitter for brain. Activation of NMDA receptors permits Ca2+ entrance in cytosol which causes synaptic plasticity [32]. However, over stimulation of the receptor leads to the loss of synaptic function and eventually causes death of neurons which is a cause of Alzheimer’s disease [33]. Moreover, it has been suggested that NMDA reception activation triggers the production of amyloid plaques [34]. Ketamine is an NMDA receptor blocker but it causes cognitive damage in higher concentration as evident in the present study. This finding is in line with previous studies [20,21]. However, several other studies in animal model showed that ketamine higher dose do not cause neuronal damage [35].
Antioxidant has been one of the popular therapeutic choices to Alzheimer's patients. Antioxidants have been able to improve memory function as well as motor coordination in various studies[36,37]. Antioxidant complex usually contains carotenoids, vitamin C and E. Betacarotene protects tissues and cells from oxidative stress. Carotenoids are responsible for scavenging of reactive oxygen species [38]. Moreover, Carotenoids prevent cognitive decline and Alzheimer's disease progression [39]. Additionally, Cognitive and motor development favorably get influenced by dietary carotenoid n early childhood [40]. On the other hand, treating animal models of Alzheimer's disease with vitamin C could prevent the production of amyloid plaques [41]. Usually high vitamin C concentrations in blood are related with improved cognitive function and a reduced risk of cognitive impairment [42]. Vitamin E is an antioxidant that prevents protein alkylation and neutralizes free radicals [43]. Individuals with Alzheimer's disease have been found to have lower vitamin E amounts in their blood. Decreased plasma levels have also been related with an increased chance of developing Alzheimer's disease [44]. Furthermore, In animals with spinal cord damage, Vitamin C and vitamin E administration greatly enhanced motor function recovery and lack of dietary Vitamin C and vitamin E induces motor impairment [45].
This study aligns with the fact that low dose of ketamine (10mg/kg) blocks cognitive and motor coordination degradation. This finding is in line with findings of other studies which suggested that by reducing reactive oxygen species ketamine reduces oxidative stress and protect neuronal death [20,21,46]. In addition, some other studies proposed that it gives neuroprotection effect by activating mTOR and enhancing autophagy [47,48]. On the other hand, It was evident in this study that ketamine 20mg/kg dose was negatively impacting memory as well as disrupting motor coordination in Alzheimer’s mice model. In high dose ketamine causes upregulation of NMDA receptor causing toxic accumulation of intracellular calcium following reactive oxygen species generation and apoptosis of neurons resulting in cognitive dysfunction [49]. Moreover, ketamine at higher dose causes dysfunction in afferent and neurons of the nucleus accumbens [50]. Nucleus accumbens acts as a bridge between limbic and motor systems. Thus, dysfunction at the nucleus accumbens results in motor failure [51]. The cause of such variation of activity due to dose difference is yet to be explored. Furthermore, it was observed that the combination of ketamine low dose especially 10mg/kg co-administered with antioxidant ameliorated cognitive function than that of their individual use. Therefore, further studies are recommended to understand the mechanism for such synergy between ketamine and antioxidant.
Conclusion
Higher dose of ketamine cause degradation in cognitive and motor function. Nevertheless, lower dose of ketamine improved cognitive function of Alzheimer’s mice but could not enhance motor coordination. Yet the mechanism by which it produces neuroprotective effect is to be explored by further investigation at cellular and molecular level. In addition, how antioxidant and ketamine together give enhanced cognition effects compare to their individual use generates a new research focus.
Abbreviations
NMDA: N-methyl-D-aspartate; AD: Alzheimer’s disease; APP: amyloid precursor protein; BDNF: brain-derived neurot-rophic factor; ITI: inter-trial interval.
Acknowledgments
This research was funded and conducted in the Pharma-cology laboratory of the Institute for Pharmaceutical Skill Development and Research, Bangladesh. The authors are thankful to the Organization for providing them with the chance to contribute to life science.
Authors’ Contributions
This work was a collaborative effort between all authors. The project was conceived, coordinated, and supervised by MNI and SMRQN. SMRQN and KN conducted the experiments and created graphical displays. KN and SMRQN took part in the writing of the manuscript. MNI and SMRQN contributed to the interpretation of data in order to obtain a scientific conclusion. The final manuscript was read and approved by the authors.
Funding
This Study did not receive any specific grants from public, commercial, or non-profit funding bodies.
Availability of Data and Materials
The datasets used and/or analysed during this research are available upon reasonable request from the corresponding authors.
Ethics Approval and Consent to Participate
All animal studies were conducted (Ref. no. IPSDRLAB/ AHCP/01/18) in compliance with the Guide for the Care and Use of Laboratory Animals, 8th edition; The National Academies Collection, as accepted by the institutional guideline for animal care.
Institute of Pharmaceutical Skill Development and Research, Bangladesh (project approved on 10/03/2022) authorized the experimental design with an Institutional Ethical Committee Clearance (Ref. No. IPSDRLAB/IECC/03/22).
Consent for Publication
Not applicable.
Competing Interests
Before submitting the article, all authors were in accord and there were no conflicts of interest.
References
- Brodaty H, Breteler MMB, Dekosky ST, et al. The world of dementia beyond 2020. J Am Geriatr Soc. 2011;59(5):923-927. DOI PubMed Google Scholar Link
- Gaugler J, James B, Johnson T, Reimer J, Weuve J. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021;17(3):327-406. DOI Link
- Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q. The Humanistic and Economic Burden of Alzheimer’s Disease. Neurol Ther. 2022;11(2):525-551. DOI PubMed PMC Google Scholar Link
- Durst F, Tropea C. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595-608. DOI PubMed PMC Google Scholar Link
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101-112. DOI PubMed Google Scholar Link
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3(4):519-526. DOI PubMed Google Scholar Link
- Tripathi T, Kalita P. Synergistic Effect of Amyloid-β and Tau Disrupts Neural Circuits. ACS Chem Neurosci. 2019;10(3):1129-1130. DOI PubMed Google Scholar Link
- Busche MA, Hyman BT. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci. 2020;23(10):1183-1193. DOI PubMed Google Scholar Link
- Mohan G, Man Z, Yang L. Genes associated with Alzheimer’s disease: an overview and current status. Dove Press. 2016;11:665-681. DOI PubMed PMC Google Scholar Link
- Armstrong RA. Risk factors for alzheimer disease. [Factores de riesgo para la enfermedad de Alzheimer]. Brain and Nerve. 2019;57(2):87-105. DOI PubMed Google Scholar Link
- Kinsella M, Monk C. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimers Dement. 2012;23(1):1-7. DOI PubMed PMC Google Scholar Link
- Vinicius M, De Mello C, Vieira L, Cruz de Souza L, Gomes K, Carvalho M. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26(33):1-11. DOI PubMed PMC Google Scholar Link
- Signs and Symptoms of Alzheimer’s Disease and Dementia | Dementia Support. Accessed November 18, 2022. Link
- Physical symptoms of alzheimer's disease. WebMD. https://www.webmd.com/alzheimers/guide/alzheimers-body. Published 2022. Accessed November 20, 2022. Link
- Iturria-Medina Y, Sotero RC, Toussaint PJ, et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7(May). DOI PubMed PMC Google Scholar Link
- Anwal L. a Comprehensive Review on Alzheimer’S Disease. World J Pharm Pharm Sci. 2021;10(7):1170. Google Scholar Link
- Feng Y, Wang X. Antioxidant therapies for Alzheimer’s disease. Oxid Med Cell Longev. 2012;2012. DOI PubMed PMC Google Scholar Link
- Ignácio ZM, Réus GZ, Arent CO, Abelaira HM, Pitcher MR, Quevedo J. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol. 2016;82(5):1280-1290. DOI PubMed PMC Google Scholar Link
- Sleigh J, Harvey M, Voss L, Denny B. Ketamine - more mechanisms of action than just NMDA blockade. Trends Anaesth Crit Care. 2014;4(2-3):76-81. DOI Google Scholar Link
- Phensy A, Duzdabanian HE, Brewer S, et al. Antioxidant treatment with N-acetyl cysteine prevents the development of cognitive and social behavioral deficits that result from perinatal ketamine treatment. Front Behav Neurosci. 2017;11(June):1-17. DOI PubMed PMC Google Scholar Link
- Bove M, Tucci P, Dimonte S, Trabace L, Schiavone S, Morgese MG. Postnatal Antioxidant and Anti-inflammatory Treatments Prevent Early Ketamine-Induced Cortical Dysfunctions in Adult Mice. Front Neurosci. 2020;14(November):1-12. DOI PubMed PMC Google Scholar Link
- Farber NB, Wozniak DF, Price MT, et al. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: Potential relevance to schizophrenia? Biol Psychiatry. 1995;38(12):788-796. DOI PubMed Google Scholar Link
- Weckmann K, Deery MJ, Howard JA, et al. Ketamine’s antidepressant effect is mediated by energy metabolism and antioxidant defense system. Sci Rep. 2017;7(1):1-11. DOI PubMed PMC Google Scholar Link
- Yadang FSA, Nguezeye Y, Kom CW, et al. Scopolamine-Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. Int J Alzheimers Dis. 2020;2020:1-10. DOI PubMed PMC Google Scholar Link
- Ma MX, Chen YM, He J, Zeng T, Wang JH. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience. 2007;147(4):1059-1065. DOI PubMed Google Scholar Link
- Nunez J. Morris water maze experiment. J Vis Exp. 2008;(19):12-13. PubMed PMC Google Scholar Link
- Deacon RMJ. Measuring motor coordination in mice. J Vis Exp. 2013;(75):1-8. DOI PubMed PMC Google Scholar Link
- Liu Y, Sun JD, Song LK, et al. Environment-contact administration of rotenone: A new rodent model of Parkinson’s disease. Behav Brain Res. 2015;294:149-161. DOI Google Scholar Link
- Noble W. Europe PMC Funders Group Advances in tau-based drug discovery. 2011;6(8):797-810. DOI PMC Google Scholar Link
- Sheng M, Sabatini BL, Su TC. Synapses and Alzheimer ’ s Disease. Cold Spring Harb Perspect Biol. Published online 2018:1-18. DOI PubMed PMC Google Scholar Link
- Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother. 2011;11(5):665-676. DOI PMC Google Scholar Link
- Kodis EJ, Choi S, Swanson E, Ferreira G, Bloom GS. N-methyl-D-aspartate receptor–mediated calcium influx connects amyloid-β oligomers to ectopic neuronal cell cycle reentry in Alzheimer’s disease. Alzheimer’s Dement. 2018;14(10):1302-1312. DOI PubMed PMC Google Scholar Link
- Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383-400. DOI PubMed Google Scholar Link
- Malinow R. New developments on the role of NMDA receptors in Alzheimer’s disease. Curr Opin Neurobiol. 2013;22(3):559-563. DOI PubMed PMC Google Scholar Link
- Schwenk ES, Pradhan B, Nalamasu R, et al. Ketamine in the Past, Present, and Future: Mechanisms, Metabolites, and Toxicity. Curr Pain Headache Rep. 2021;25(9). DOI PubMed Google Scholar Link
- Madiha S, Batool Z, Tabassum S, et al. Quercetin exhibits potent antioxidant activity, restores motor and non-motor deficits induced by rotenone toxicity. PLoS One. 2021;16(11). DOI PubMed PMC Google Scholar Link
- Adebiyi OE, Olayemi FO, Olopade JO, Tan NH. Βeta-sitosterol enhances motor coordination, attenuates memory loss and demyelination in a vanadium-induced model of experimental neurotoxicity. Pathophysiology. 2019;26(1):21-29. DOI PubMed Google Scholar Link
- Balbuena E, Cheng J, Eroglu A. Carotenoids in orange carrots mitigate non-alcoholic fatty liver disease progression. Front Nutr. 2022;9(September):1-16. DOI PubMed PMC Google Scholar Link
- Yuan C, Chen H, Wang Y, Schneider JA, Willett WC, Morris MC. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am J Clin Nutr. 2021;113(1):200. DOI PubMed Google Scholar Link
- Fifield L, Khan N, Walk A, Keye S, Rosok L, Sarma R. Skin Carotenoids Are Related to Cognitive and Motor Skills Among Toddlers. Curr Dev Nutr. 2022;6(Supplement_1):59-59. DOI Google Scholar Link
- Alzheimer’s Disease | Linus Pauling Institute | Oregon State University. Accessed November 24, 2022. Link
- Alzoubi KH, Shatnawi AF, Al-Qudah MA, Alfaqih MA. Vitamin C attenuates memory loss induced by post-traumatic stress like behavior in a rat model. Behav Brain Res. 2020;379. DOI PubMed Google Scholar Link
- Shuaib F, Kimbrough D, Roofe M, McGwin Jr G, Jolly P. Ascorbic Acid Promotes Detoxification and Elimination of 4- Hydroxy-2(E)-nonenal in Human Monocytic THP-1 Cells. Chem Res Toxicol. 2009;22(5):1-7. DOI PubMed PMC Google Scholar Link
- Browne D, McGuinness B, Woodside J V., McKay GJ. Vitamin E and Alzheimer’s disease: what do we know so far? Clin Interv Aging. 2019;14:1303. DOI PubMed PMC Google Scholar Link
- Pierce MR, DiAsio DL, Rodrigues LM, Harrison FE, May JM. Combined Vitamin C & E deficiency induces motor defects in gulo−/−/SVCT2+/− mice. Nutr Neurosci. 2013;16(4):160-173. DOI PMC Google Scholar Link
- Wang R, Zhang Z, Kumar M, Xu G, Zhang M. Neuroprotective potential of ketamine prevents developing brain structure impairment and alteration of neurocognitive function induced via isoflurane through the PI3K/AKT/GSK-3β pathway. Drug Des Devel Ther. 2019;13:501-512. DOI PubMed PMC Google Scholar Link
- Fan JC, Song JJ, Wang Y, Chen Y, Hong DX. Neuron-protective effect of subanesthestic-dosage ketamine on mice of Parkinson’s disease. Asian Pac J Trop Med. 2017;10(10):1007-1010. DOI PubMed Google Scholar Link
- Choudhury D, Autry AE, Tolias KF, Krishnan V. Ketamine: Neuroprotective or Neurotoxic? Front Neurosci. 2021;15(September):1-9. DOI PMC Google Scholar Link
- Liu F, Patterson TA, Sadovova N, et al. Ketamine-induced neuronal damage and altered N-methyl-d-aspartate receptor function in rat primary forebrain culture. Toxicol Sci. 2013;131(2):548-557. DOI PubMed Google Scholar Link
- Razoux F, Garcia R, Léna I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology. 2007;32(3):719-727. DOI PubMed Google Scholar Link
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Rev. 2000;31(2-3):330-341. DOI PubMed Google Scholar Link
Update History
| Revision Number | Date | Details Of Changes |
|---|---|---|
| 1 | 2022-11-25 | Original Article; published at its accepted version (Reference Number: PPD/MIN/22212A). |
 Pharmacotherapy & Pharmascience Discovery
Pharmacotherapy & Pharmascience Discovery
